

If we were to dissect and analyze a copper anode, we would discover four basic factors contribute to its quality. They are: raw material, grain structure, phosphorus content, and cleanliness. Raw Material. The raw material for a copper anode comes from one of two places; the copper is either refined or scrap material. Refined copper comes in the form of a copper cathode, named so because copper is electrolytically refined (plated out of solution). In this process, blister copper (approximately 95% copper) is dissolved anodically and high purity copper metal is plated slowly on a cathode with the impurities falling to the bottom of the plating tank as sludge. The impurities in the sludge include iron, sulfur, tellurium, selenium, gold, silver, zinc, arsenic, and others. The resulting cathode is 99.95% pure copper and can now be melted to form anodes.
Scrap copper is generated daily around the world from such sources as utility wires, telephone wires, transformers, water tubes, buss bars, and other sources. This material is usually grouped and sorted by a scrap dealer. The disadvantage of scrap metal is its potential for contamination. All of the previously mentioned sources contain steel, tin, silver, and/or lead. The metals can also be mixed when sorting, and copper alloys can be mistaken for pure copper.
Some metallic impurities in copper anodes can be dissolved and plated. These dissolved impurities will increase in concentration over time, resulting in a bath saturated with metallic contaminants. Plated impurities can cause stress and roughness on the plated part, which, in turn, cause difficulties in plating and process control, higher additive consumption (brightener) and scrap parts. These impurities usually go undetected until production problems appear because they are not normally examined in routine analysis. Grain Structure. The grain structure is the most important characteristic of copper anodes; this structure is completely determined by the process used to manufacture the anodes, which we will now explore. Casting of copper anodes is the simplest and least costly method; however, cast copper anodes do not exhibit the fine, uniform grain structures necessary for superior plating. Higher casting speeds will increase production and reduce costs, but will also result in larger grain size, and, therefore, inferior grain structure.
Treatment is required subsequent to casting in order to produce copper anodes with a fine grain structure. This usually involves hot rolling, hot extruding, or hot forging. When the copper is formed under high temperature and pressure the grain structure is reformed and becomes fine and uniform. It should be noted that copper used for a variety of functions, other than anodes, require fine grain structures (wire, buss bars, copper foil and sheets).
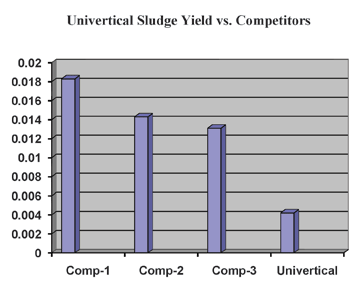
Overall, copper anodes containing higher impurities and without a fine grain structure will dissolve unevenly, have a higher sludge yield, result in lost copper dropping into solution, and have the potential to increase the roughness of plated parts. The results below show the sludge weight left in solution after 30 minutes of plating at 15ASF with four different types of copper anode balls. Filtered Solution (Weight of Sludge). Phosphorus content. Phosphorus content for acid copper plating is ideally maintained between 0.040 and 0.065%. Metallurgically, impurities are located at grain boundaries. Therefore, phosphorus (required for acid copper plating) is most evenly distributed in fine grain anodes. Typically, a black film develops as a result of the phosphorus in the anode. This film keeps fine pieces of copper from falling off of the anode as sludge and also retards corrosion of the anode. Anodes with large grain structures will develop a black film, but this film will not be adherent and quickly turn into sludge. The sludge now contains usable copper (lost metal) that can plug up anode bags and fall into solution causing roughness on plated parts. Cleanliness. Manufacturing oils, oxidation, and burrs must be removed from copper anodes. Copper oxidizes when it freezes from the molten state or when heated for rolling, forging, or extruding. This oxidation must be removed either by mechanical abrasion or acid pickling followed by neutralization. Mechanical methods are preferred because there is no residue.
In the manufacture of bar anodes sawing, drilling, and tapping oils are used to lubricate the tooling. The oils are applied to the copper as well as the tooling. Proper washing and rinsing removes these residual oils or coolants (organic compounds) which would contaminate the plating bath.
Anode nuggets are typically made by cutting copper rod into various lengths. This shearing process creates burrs and sharp edges that can drop into tanks or cause the nuggets to bridge or nest in titanium mesh baskets, causing voids of anode, uneven plating distribution, and anode polarization. In addition, burrs and sharp edges are hazards to the operators handling the material. Anodes should be inspected carefully to determine that the material has come from the manufacturer ready for use with no pretreatment necessary.
Anode balls are formed by either cold forging (heading) or hot roll forging copper rod of various thickness. Copper wire being headed should preferably already have a fine grain structure. Normally copper balls that are hot roll forged will have a post-treatment procedure to ensure they will have a fine grain structure. SUMMARY Overall, there are several characteristics that determine the quality of the copper anodes manufacturing process. These include: purity of copper, fine grain structure, uniform phosphorus content and distribution, and properly cleaned and anodes packaged and ready for use. Therefore, the quality of copper anodes should be very important to electroplaters because properly manufactured anodes will: extend bath life; have less metallic contamination; produce parts without roughness; have less sludge; and require less maintenance. BIO Chuck Walker, the son of the Univertical’s founder and currently serving as CEO, began his career at Univertical in 1975 working in the manufacturing department. In the following years, Walker worked in a variety of positions of increasing responsibility and importance in production and management. He has held leadership positions in manufacturing, maintenance, process engineering, accounting and sales during his tenure at Univertical. Under Walker’s leadership, the company has achieved strong sales growth and profitability, driven by new product designs, manufacturing process improvements and expansion into new markets. His major accomplishments include the development of the graphite basket for corrosive acid metal plating solutions and the three-axis technology utilized today for anode manufacturing. In 2003, Walker led the establishment of the Suzhou facility, a subsidiary that has experienced double-digit revenue growth each year since its inception. As CEO, Mr. Walker is responsible for the strategic direction of the company and the overall success and operation of Univertical.





