

There is increasing interest in utilizing mixed metal oxide (MMO) electrodes in a wide variety of plating applications. These dimensionally stable electrodes have very high catalytic activity and provide much higher throwing power and, hence, better plating distribution. This is particularly important for areas of the plated part that have an unfavorable aspect ratio or deep recesses. While the high catalytic activity is desirable, it can have consequences for the plating bath. In some instances, plating chemicals can be decomposed at the anode, thereby increasing chemical costs. Alternatively, the MMO coatings can be adversely affected by additives in the bath and the lifetime might be compromised.
MMO coatings were originally developed by Henri Beer in the late 1950’s after extensive development. The original effort was directed toward replacement of graphite anodes that were used in the electrochemical generation of chlorine and caustic soda. Due to dramatic reduction in power costs and the dimensional stability of MMO anodes, these rapidly displaced graphite and are now used exclusively in chlorine generation. Since then, several major market sectors have adopted these dimensionally stable MMO electrodes. They are now used in very large copper electrowinning plants and have been broadly adopted for plating printed circuit boards; unlike soluble anodes, they can throw into deep vias and make very fine, highly defined lines for high-density electronics.
With an ever-increasing stability of anode coatings, their application continues to spread, allowing purification of waste for swimming pools, making chlorine on site for water treatment plants, and purifying seawater used in harvesting oil from offshore oil platforms. Due to ever-increasing demands in the manufacture of electronics, they are used for making ultra pure water via a process called electro-deionization.
MMO electrodes are generally described as the combination of a platinum group metal oxide (PGMO) and a valve metal oxide. Henri Beer found that these combinations provided unique stability and adherence to the titanium substrate to which they are applied. This occurs in large part because these make “solid solutions” and actually grow into the surface of the titanium substrate. This provides the source of adhesion between the precious metal paints and the surface of the titanium.
Originally, chlorine generation was the only application that exploited the excellent characteristics of MMO electrodes, so generally only one formulation was offered. As the use spread, however, so did the development of alternate coatings that were tailored for good performance and lifetime. Generally, the selection of PGMO and valve metal oxide is based on the operating conditions. For chlorine evolution, the formulation is a combination of ruthenium oxide and titanium oxide. Oxygen evolution, as in the plating industry, requires the use of a mixture of iridium and tantalum oxides since ruthenium is unstable in the oxygen evolution region. The ratio of PGMO to valve metal oxide can be varied to improve lifetime or to maximize electrochemical activity.
HOW DO MMO COATINGS WORK? The commercial success realized by MMO electrodes is largely due to their excellent electrocatalytic properties and high surface area. Electrocatalysis is the ability of the electrode to influence the rate of electrochemical reactions, i.e., reactions such as the evolution of chlorine, hydrogen and oxygen. They will also oxidize organic compounds (such as brighteners and levelers) and inorganic ions such as iron. In an electrochemical reaction, molecules in the electrolyte migrate to the surface of the electrode, where they are absorbed or adsorbed to allow transfer of electrons. These interactions reduce the energy required to drive the reaction, lowering the electrode potential and, hence, the overall cell voltage. Consequently, the electrical power consumed by the process is reduced.
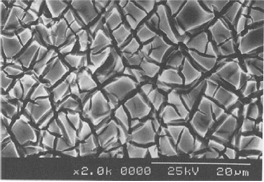
The surface area of a commercially available, mixed metal oxide electrode is high, due to the microscopic cracks, crevices and pits created by the method of manufacture. The high surface area is an important factor in an electrochemical process, since it determines the applied current density, i.e., current per unit area. The electrode potential is dependent upon the real current density used in the process and a lower current density results in a lower electrode potential and, therefore, a lower cell voltage. The typical surface morphology of a mixed metal oxide electrode can be seen in a SEM photograph (Figure 1), which clearly shows the “mud-cracked” appearance now associated with these electrodes. It contrasts sharply with the relatively smooth surfaces of commercially available platinum, platinized titanium and lead alloy electrodes used in the plating industry.
The catalytic layer of Water Star’s Suppress anode has a unique morphology, significantly different from that of commercially available mixed metal oxide electrodes. This is clearly shown by the SEM photograph of the surface of a Suppress anode (Figure 2). In contrast to a typical mixed metal oxide electrode, the Suppress coating is relatively smooth, more compact and, therefore, less porous, and yet provides the necessary electrocatalytic activity—albeit the surface area is somewhat reduced over a “standard” MMO electrode. The formulation of Suppress is designed to reduce the catalytic activity of the electrode by limiting the amount of PGMO at the electroactive surface while maintaining electrical conductivity throughout the coating layer. The catalytic coating of the Suppress anode can be tailored to effectively control the potential at which the electrode operates. In this way it is possible to inhibit the oxidation of additives, such as levelers and brighteners, when used in plating and surface finishing processes. Or in another formulation, it can reduce chloride evolution in high chloride environments by more than 70%. This can dramatically reduce generation of noxious chlorine from chloride-containing baths.
TEST PROTOCOL Tests were done to simulate actual operating conditions in the field, and the actual operating parameters were those specified for each of the specific MacDermid plating processes. The solutions were electrolyzed for 60 amp-hours. When soluble anodes were used, they were electrically driven independently from the MMO. Testing for the presence of hexavalent chrome was done immediately after completion of the tests. In order to limit the amount of chrome removed from the bath, a very small cathode was used in combination with the anodes. Special test cells were built to limit the electrolyte volume and thus minimize the test time.
PLATING CHEMISTRY Three different plating baths were utilized to better understand the relationship between anodes and plating chemistry.
Hexavalent chromium is still the industry standard for use in decorative plating applications. This process requires the use of tin-lead anodes as the primary anode in the system. In applications requiring plating into deep recesses and extreme low current density areas, the use of mixed metal oxide auxiliary anodes may also be employed.
While several different hexavalent chromium systems exist, a triple-catalyst system was used for the purpose of this testing. This system operates using a mixture of sulfate, fluoride and organics in addition to chromic acid and proprietary catalysts to achieve the desired finish.
Trivalent chromium is gaining more popularity in the decorative plating arena. Two different types of trivalent chromium systems exist. A sulfate-based electrolyte may be utilized which involves the use of mixed metal oxide anodes. A chloride-based electrolyte is also an option and operates with graphite anodes. Both of these systems were incorporated into this testing.
ELECTRICAL TEST SET-UP Figure 3 shows the electrical test set-up for the laboratory studies. Four test stations in all were utilized.
Test Matrix Several anodes were tested in each of the three plating baths. Each bath had a “control” anode representing the anode that would normally be used in the decorative plating operation. The standard mixed metal oxide anode was the control for the hexavalent and sulfate-based trivalent chrome baths, while a graphite anode served as the control for the chloride-based trivalent chrome system. All baths were run at their recommended anode current densities. The trivalent chromium baths were tested for the presence of hexavalent chromium immediately after the testing was completed. A fresh plating solution was used for each of the test conditions and each solution was prepared according to recommended Technical Data Sheet parameters. All of the plating test conditions are outlined in Table 1. Table 1. Test Conditions
| PLATING BATH | ANODE | ADODE CURRENT DENSITY | COMMENTS |
| Hexavalent chrome | Standard MMO | 150 ASF | |
| Suppress MMO #1 | 150 ASF | ||
| Suppress MMO #2 | 150 ASF | ||
| Trivalent chrome - sulfate | Standard MMO | 50 ASF | Test for Hex |
| Suppress MMO #1 | 50 ASF | Test for Hex | |
| Suppress MMO #2 | 50 ASF | Test for Hex | |
| Trivalent chrome - chloride | Graphite | 40 ASF | Test for Hex |
| Standard MMO | 40 ASF | Test for Hex | |
| Standard MMO #1 | 40 ASF | Test for Hex | |
| Standard MMO #2 | 40 ASF | Test for Hex | |
| Standard MMO #3 | 40 ASF | Test for Hex |
ANALYTICAL DATA AND RESULTS A variety of parameters were analyzed for each of the three plating systems. The parameters were chosen based on standard recommended analytical procedures for each chrome bath. The data can be seen in Tables 2-4.
Table 2. Hexavalent chromium test results
| Hexavalent Chrome | Standard MMO | Standard MMO #1 | Standard MMO #2 |
| Chromic acid (oz/gal) | 30.73 | 29.99 | 29.50 |
| Sulfate (oz/gal) | 0.02 | 0.02 | 0.01 |
| Chrome: Sulfate | 1536 | 1500 | 2950 |
| Trivalent Chrome (oz/gal) | 0 | 0 | 0 |
| Available Fluoride (ppm) | 70 | 81 | 62 |
| Total Fluoride (ppm) | 203 | 218 | 248 |
| Copper (ppm) | 0 | 0 | 0 |
| Iron (ppm) | 0 | 0 | 0 |
Table 3. Sulfate-based trivalent chromium test results
| Sulfate Trivalent Chrome | Standard MMO | Suppress MMO #1 | Suppress MMO #2 |
| Trivalent Chrome (oz/gal) | 1.15 | 1.15 | 2.33 |
| Additive 1 (g/L) | 369.38 | 389.83 | 382.52 |
| Specific Gravity | 1.19 | 1.207 | 1.206 |
| Surface Tension (dynes/cm2) | 36.34 | 36.8 | 38.18 |
| pH | 3.43 | 3.48 | 3.39 |
| Additive 2 (% by vol) | 22.62 | 37.23 | 26.69 |
| Nickel (ppm) | 0 | 0 | 0 |
Table 4. Chloride-based trivalent chromium test results
| Chloride Trivalent Chrome | Graphite Anode | Standard MMO | Suppress MMO #1 | Suppress MMO #2 | Suppress MMO #3 |
| Trivalent Chrome (oz/gal) | 2.97 | 2.79 | 2.72 | 2.39 | 2.97 |
| Specific Gravity | 1.195 | 1.205 | 1.2 | 1.2 | 1.19 |
| pH | 2.12 | 2.21 | 2.34 | 2.34 | 2.43 |
| Additive 1 (% by vol) | 3.21 | 0.56 | 0.43 | 0.74 | 3.67 |
| Surface Tension (dynes/cm2) | 55.66 | 58.88 | 64.86 | 69.69 | 58.88 |
A comparison of the results for each of the test conditions shows that there are alternatives to the anodes that are currently being utilized in each of the chromium plating baths today. The analytical results for hexavalent chromium show that the Suppress MMO #1 performs comparably to the Standard MMO anode currently used. Both Suppress MMO anodes tested with the sulfate-based trivalent chromium baths provided suitable results. Additionally, the Suppress MMO #3 anode performed well in comparison to the graphite anode traditionally used in the chloride-based trivalent chromium system.
CONCLUSIONS AND FURTHER TESTING MacDermid has a commitment to provide the best state-of-the-art chemicals to the plating industry and has begun an effort to study the interactions between MMO anodes and various plating baths. The initial data obtained in this study is promising and warrants further testing on chromium and other plating systems. Specifically, testing of Suppress anodes will be performed on semi-bright nickel systems as a comparison to the MMO auxiliary anodes currently being used in some applications. Lifetime tests are under way for the Suppress anodes in chromium plating solutions based on the favorable results from this study. Additional formulations of the Suppress anode are also being developed to reduce the formation of hexavalent chromium in trivalent chromium plating systems.
ABOUT THE AUTHORS Chrissy Hnetinka is a marketing manager at MacDermid, focusing her efforts on decorative products and fashion finishes. She has more than 10 years of industry experience, including research and development, technical service and marketing. She has been intimately involved with the development of new trivalent chromium technologies and works closely with anode manufacturers to optimize chemical processes for increased efficiency and productivity.
Marilyn Niksa has more than 35 years of experience in the electrochemical industry and is listed as inventor of more than 35 patents. She presently serves as president of Water Star, Inc., a manufacturer of precious metal coated anodes. Along with her partner, Andy Niksa, who also has decades of experience in the industrial electrochemical industry, she is heavily involved in the development of proprietary electrochemical technologies, including new electrode coatings, hypochlorous acid generators, and systems that generate hydrogen peroxide.





