



A failure analysis to determine the root cause of the failures was performed. The manufacturer of the boxes, the coating application method, any specific information on the coatings, and the service environment were initially unknown. However, we determined much of this critical information through a systematic investigation using a combination of advanced analytical techniques. Based on the information collected, the root cause of the paint failure was successfully determined. This paper presents our failure analysis methodology and the step-by-step approach to this particular case.
A site inspection was initially performed where the recalled boxes had been stored. The boxes were comprehensively photo-documented. Initial photo-documentation is critical step in any failure analysis investigation because it is often difficult or impossible to return to the site to gather potentially missed details and the material of interest might be further modified, destroyed, or otherwise lost.
Taking photographs for failure analysis is not as straightforward as it sounds. Weeks or months after the inspection one might want to review the images, but cannot remember which photograph refers to which steel box, the location of the paint failure on the box, the physical relationship of one failure to another, etc. Therefore, taking photographs must be carried out methodically—and with good documentation.
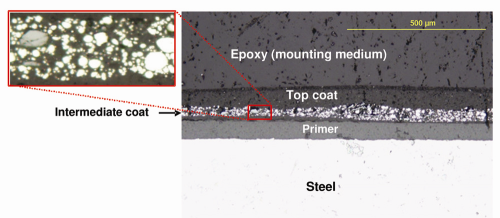
All of the boxes inspected had an external green top coat with an interior gray top coat. The coating failures were easily detectable by visual examination. A significant amount of paint blisters were observed on the external surfaces, with fewer blisters internally; moreover, the blisters appeared to be more pronounced near the edges and corners. The coating had cracked and delaminated from the underlying steel, mainly on the external surfaces near the corners and joints; corrosion of the steel was observed at areas where the coating had delaminated. Figures 1, 2, and 3 illustrate these failures.
Microscopic Inspection of the Coating Samples. To identify the fundamental causes of the coating failures, we first collected coating samples from the boxes and performed a series of microscopic inspections. This allowed us to obtain critical information on what coating system had been applied, what surface preparation had been carried out, what coatings were used, the method of coating application, and whether the selection of the coatings was appropriate for the environment in which the boxes were exposed.
Samples were not taken where delamination was observed and where the underlying steel was already severely rusted. Instead, because it was necessary to examine the interface between the coatings and the non-rusting steel substrate, areas were selected where a portion of the coating was just starting to fail but the adjacent areas were still in good condition. These samples would also allow us to determine if the steel had been galvanized or received a zinc-rich primer.
Because we believe these boxes were manufactured for a corrosive un-sheltered outdoor environment, it was reasonable to expect that the coating system would comprise multiple layers. To get a quick overview of the coating system, we prepared transverse section samples for metallographic and scanning electron microscopic (SEM) examination. Three separate boxes were randomly selected (identified as Boxes A, B, and C). Coated steel samples were cut from the three boxes using a band saw and cold water to prevent the coating from overheating. The samples were mounted in epoxy resin cured at ambient temperature in order to avoid sample overheating and possible degradation of the coating/steel interface. Once the mounts were cured, they were polished and prepared for microscopic observation and analysis.
Coating System–Where is the Zinc? We examined the cross-sections using an optical microscope (OM) with a magnification range of 5× to 100×. Interestingly, the three boxes were not coated in the same manner, although they had similar appearances.
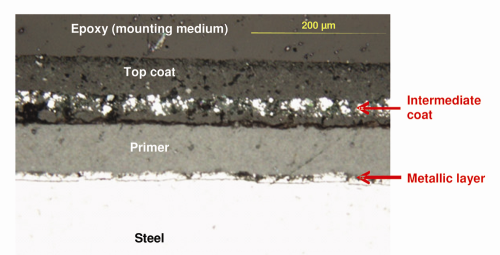
Coating System: Boxes A and C. The microscopic analysis revealed that primer had been applied directly to the steel on Box A and Box C, followed by an intermediate coat containing metallic pigments, with a green top coat. When viewed under a microscope, bright reflection of the pigments in the intermediate coat indicated that the pigments were metallic, because non-metallic pigments generally appear dull. The thickness of the intermediate and top coats varied significantly, within a sample as well as across samples. Figure 4 shows a representative microscopic image of the external surface from Box A. The interior steel surface of Box A and Box C had only one layer of primer (image not shown).
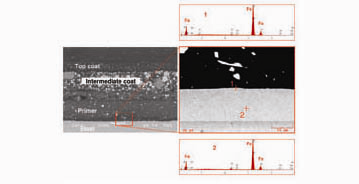
A galvanized zinc layer on Box A and Box C was not visible, even at a magnification of 100×. Had a galvanized zinc layer been present, it would have been easily identifiable. A scanning electron microscope (SEM) was used to confirm the absence of zinc. The SEM forms images by scanning the area of interest with a very fine electron beam and allows imaging at a much higher magnification, with better resolution than an optical microscope. Modern SEMs are usually equipped with an energy-dispersive X-ray spectroscopy (EDS) system that permits determination of the elemental constituents of an area as small as a micrometer. Figure 5 represents EDS spectra collected from two spots on Box A: Spot 1, 10 µm below the steel/primer interface; Spot 2, at the interface between the steel and the primer. EDS spectra from both spots showed strong iron (Fe) peaks (from the steel) but no zinc. Similar results were obtained from Box C. The lack of a zinc peak confirmed that the steel on Boxes A and C were not directly treated with either galvanized zinc or a zinc-rich primer.
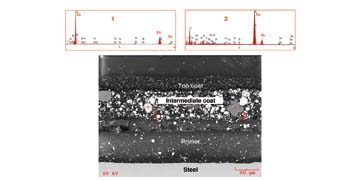
Despite the absence of a galvanized zinc layer on Box A and Box C, we found zinc in the intermediate coat (Fig. 6). EDS spectra obtained from a “brighter” pigment (Spot 1) and a “darker” pigment (Spot 2) in the intermediate coat confirmed that they were zinc-rich and iron-rich, respectively. This confirmed the intermediate coat was, in fact, a zinc-rich paint.
It is likely the manufacturer of the boxes assumed a zinc-rich intermediate coat would provide cathodic protection to the underlying steel. However, in order for the zinc-rich coating to provide cathodic protection, or to act as an inhibitive layer, it must be applied directly to the steel surface. The lack of cathodic protection was the major reason for the severe corrosion on Box A and Box C.
Figure 7 shows the OM and SEM pictures taken from an area on Box A, where contaminants between the intermediate coat and the primer were noticed. The EDS spectrum of the contaminants exhibited a strong chlorine (Cl) peak, and although the source of chlorine could not be identified, we suspect that the boxes may have been exposed to a marine or coastal atmospheric environment. In such a case, the manufacturer should have been expected to thoroughly wash down the primed surfaces with fresh water to remove the chloride deposits before applying the intermediate and top coats. This is a common industrial practice where contaminants deposit during storage of primed parts.
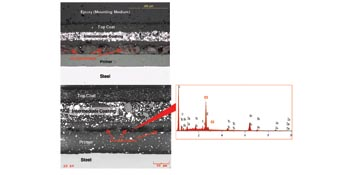
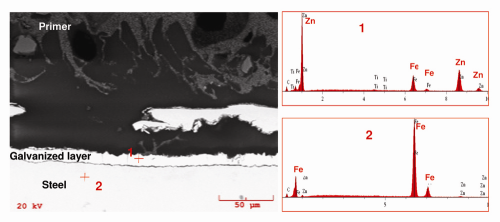
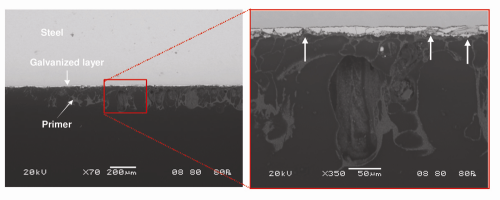
Coating System: Box B. In contrast to Box A and Box C, a metallic layer was found directly applied to the steel surface in Box B (Fig. 8). EDS confirmed the metallic layer was high in zinc (Fig. 9). Furthermore, the coexistence of zinc and iron peaks on the EDS spectra from the metallic layer suggested it was likely a galvanized zinc layer. The interior surface of Box B was protected with a zinc layer followed by one layer of primer (image not shown). Despite the galvanizing, Box B still experienced paint delamination and steel corrosion. This suggested that the galvanized layer was not able to provide sufficient protection to the underlying steel surface.
Air Bubble Entrapment in Primer Layer: Box B. Optical microscope examination of the Box B cross-sectioned samples disclosed a notable difference in coating appearance, as compared with Box A and Box C. Extensive air bubbles (or pockets) were found in the primer on Box B. The air bubbles caused severe deformation of the primer and contributed to its delamination from the galvanized surface, as shown in Figure 10. The extent of zinc damage was particularly pronounced under the areas with extensive air entrapment (Fig. 11).
Air bubbles in the primer would have significantly reduced the primer’s ability to withstand atmospheric moisture penetration. Failure occurred between the primer and subsequent coats, contributing to corrosion of the boxes. The galvanized layer was extensively consumed under the failed primer and exhibited cracks and loss of material; the galvanizing provided poor coverage of the underlying steel.
Chemical Analysis and Root Cause of Coating Failure: Box B. Fourier transform infrared spectroscopy (FTIR) on the coating samples was performed to explain the root cause of the coating failure on Box B and the different appearance of the primer compared with Boxes A and C. FTIR is an analytical technique widely used to determine the chemical bonding and molecular structure of both inorganic and organic compounds (e.g., resins or pigments).
FTIR results showed that all three top coats (exterior surface) were urethane-based; their absorbance peaks were similar and matched a library reference for an acrylate urethane. FTIR results also showed that the same type of primer was used on Box A and Box C, but a different type was used on Box C. Using a library reference spectrum, the FTIR analysis confirmed the primer on Box A and Box C was an epoxy-based primer, but the primer on Box B was a urethane alkyd primer.
The use of an alkyd-modified primer on Box B was surprising, because it is well accepted within the industry that an alkyd-based primer should never be applied directly over galvanized surfaces. In the presence of atmospheric moisture, the zinc corrosion products caused the alkyd to saponify and break down. Homeowners frequently see this when alkyd paints are applied onto galvanized gutters and downpipes. With regard to Box B, the extreme porosity of the primer (i.e., air bubbles) was likely the result of saponification, and this led to the complete deterioration of the primer.
The composition of a urethane alkyd primer on Box B suggests the primer was likely a liquid rather than a powder coating. This is further supported by looking at the sharp cut edges on the steel. The microscopic images from the cut edge show that there was a layer of primer on the exterior and interior surfaces of the enclosure, but it disappeared as it approached the cut edge. Liquid coatings are known to pull away from sharp edges due to the surface tension effects of the liquid at these locations. Powder coatings, on the other hand, are applied to metals using electrostatic attraction. Electrostatic charges at sharp edges and points of a metallic object are known to be accentuated. Had the primer been powder based, excess coating would have deposited at the cut edges. Consequently, we concluded that the primer on Box B was a liquid coating.
If, in fact, the urethane-alkyd primer on Box B was a liquid coating, the large pockets shown in Figures 10 and 11 could also have been due to solvent entrapment during the application process. Solvent entrapment occurs when the primer is applied too thickly and insufficient time is allowed for the solvent to flash off before applying the top coats. Excessive thicknesses of high solid coatings are not unusual and can have several causes—for example, they can arise if the spray gun is improperly set up; the solvent blend in the primer has a low vapor pressure; or the primed surface is placed in an oven before the solvents have had sufficient time to flash. The two most common probable causes for “catastrophic peeling” are due to poor surface preparation and solvent entrapment.1
Summary
A systematic failure analysis was performed on three large steel boxes in which the coating system had blistered, extensively delaminated, and subsequently corroded. These boxes were a sample from a larger batch of boxes that had been recalled due to severe corrosion.
Despite the fact that limited information on the process history was provided, we were able to determine that two out of three boxes were neither galvanized nor protected by a zinc-rich primer to provide cathodic protection. The coating system comprised an epoxy primer, zinc-rich intermediate coat followed by an acrylate urethane top coat. A zinc-rich coating should never be applied over a non-metallic coating because cathodic protection of the underlying steel can only take place if the zinc-rich coating is in direct electrical contact with clean steel.
The third box was galvanized and primed with a urethane-alkyd primer followed by an acrylate urethane top coat. Alkyd-type coatings should not be applied over zinc (galvanizing) because the corrosion products of zinc are alkaline. Alkyd-modified coatings are very sensitive to alkalinity and a saponification reaction occurs at the zinc-alkyd interface. This degrades the alkyd and causes it to peel or delaminate from the zinc alloy used for the galvanization.
The epoxy primer showed unusually severe air bubbles (or pockets) that could have been due to saponification and/or solvent entrapment. In either case, these large pockets weakened the bond between the primer and galvanized layer and allowed delamination to occur.
Our finding that chlorides were present on the surface of the epoxy primer indicated that the boxes might have been exposed to a marine or coastal atmospheric environment. Marine or coastal atmospheric corrosion is generally considered to be one of the most severe atmospheric corrosion environments and the presence of chlorides explains the severity of the corrosion.
The boxes were presumed to have been powder coated, yet microscopic and chemical analysis showed the coatings were probably applied as liquids. This was further supported by the thinning of the coatings at the sharp edges and corners of the boxes.
About the Authors
Dr. Zixiao Pan is a materials scientist and a consultant at Exponent Failure Analysis Associates in Menlo Park, Calif. She holds a PhD in materials science and engineering from Northwestern University. Dr. Pan’s areas of expertise include powder metallurgy, composites, advanced surface analysis, and microstructural characterization.
Ron Joseph is a senior managing engineer at Exponent Failure Analysis Associates in Menlo Park, Calif., and the organic coatings editor for Metal Finishing magazine. He has more than 38 years of experience in the field of paints and coatings, and his knowledge covers both liquid paints and powder coatings. Joseph has worked with solvent-based and waterborne paints covering a wide range of resins, including alkyd, epoxy, polyurethane, acrylic, polyester, vinyl, melamines, TGIC, hybrids, and nylon.
NOTES
- “Catastrophic peeling” is the industry term used to describe paint peeling over very large surfaces. When only small pieces of paint peel from the previous coat or from the substrate, the term “catastrophic” is not appropriate.






