
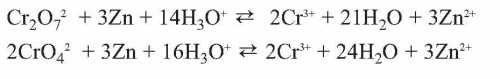
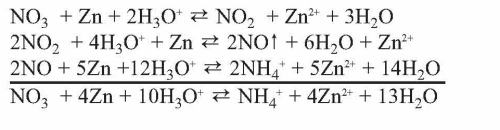







The application of hexavalent chromium for conversion coatings reflects on a long and successful history in corrosion-resistant coatings starting in the early 1930s.1 Although trivalent chromium-based conversion coatings date back as early as 1951,2 their industrial application was forced into practice by the European End-Of-Live-Vehicle (ELV) directive.3 In addition, the RoHS4 and WEEE5 directives preclude hexavelent chromium from being used in metal coatings in electrical and electronic equipment. Therefore, the use of hexavalent chromium is actually limited to a rather small number of remaining applications.
In the years preceding the legal ban of hexavalent chromium, publications concerning trivalent alternatives grew dramatically. Most papers published during this pre-regulatory era discussed trivalent chromium–based systems, and rarely were they innovative in nature. These systems were quite often said to be at least as good as the good old hexavalent chromium systems but based on the environmentally sound trivalent chromium compound. Trivalent chromates, often referred to as passivates, are less easily applied than chromates. Keeping the application parameters, such as concentration and particularly the solution’s pH, within narrow upper and lower limits became much more important when switching from hexavalent to trivalent formulations.
Simultaneous with the introduction of the new hexavalent chromium-free passivate technology, the needs of decorative and particularly corrosion protection properties have been revised in many specifications, leading to more stringent requirements for trivalent passivates. The use of sealers is often mandatory to achieve these elevated demands, especially on black passivated surfaces. Sealers, in general, mean polymer solutions or dispersions or silicate solutions that are dried on the surface, resulting in a film of either an organic or inorganic polymer on top of the conversion layer (Fig. 1). It is characteristic for a sealer layer to bear a completely different composition with regard to the underlying passivate layer. Together with the trivalent chromium-based conversion layer, the sealer layer acts as a highly efficient barrier that effectively decelerates zinc corrosion.
Sealed conversion layers achieve the most stringent corrosion protection requirements and also bear excellent decorative properties. Furthermore, by using sealers, a broad range of friction coefficients can be precisely adjusted within narrow tolerances. Despite their assets, polymer-based sealers are specifically restricted from being used in some applications. To provide excellent corrosion protection as well as advanced decorative properties in those applications, an alternative approach to enhance the conversion layer is required. To date, black finishes, in particular, do not provide reliable and sufficient corrosion protection without the application of a final finish. This final finish is not allowed to introduce any components into the coating that are not already found in trivalent chromium conversion coatings. At the same time, it needs to fulfill the high decorative and functional demands required by the automotive industry. This approach leads to the obvious question: Why not just put more chromium into the layer?
This is not a cutting-edge idea. Post-dip solutions based on hexavalent chromium were already used on black hexavalent chromium-based chromate conversion coatings, particularly on some black zinc–iron layers. These post-dip solutions mainly consisted of a hexavalent chromium source such as sodium dichromate in a dilute acidic solution.
With the restrictions on hexavalent chromium use, the logical step from hexavalent chromium post-dips to trivalent chromium–based post-dips became obvious. Just changing from hexavelent chromium chemistry to any trivalent chromium compound does not result in sufficient performance as a final finish. Surfaces achieved this way are noticeably inferior with regard to corrosion protection, as well as decorative properties. As with the change from chromates to passivates, R&D had to think about how the more difficult formulation and application of the new trivalent chromium-based post-dip generation could be achieved, circumnavigating the bluffs of trivalent chromium. Atotech has put quite a bit of effort into solving this problem. The outcome is Tridur Finish 300, a trivalent chromium–based post-dip solution, dedicated to passivated zinc, zinc–iron, and zinc–nickel alloys.
Formation Mechanism, Structure, and Properties of Conversion Layers
Chromate Conversion Coatings
Chromate conversion coatings (CCCs) in general develop on zinc surfaces in acidic hexavalent chromium-containing solutions by reduction of hexavalent chromium to trivalent chromium. In the course of this reaction, oxonium ions are consumed (see Equation 1).
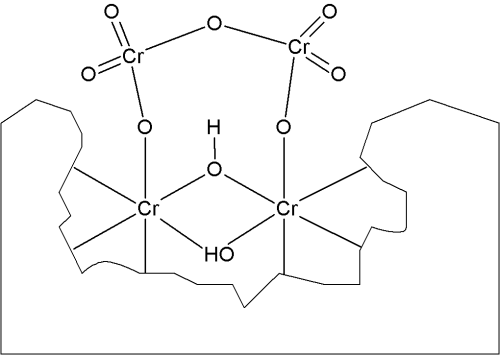
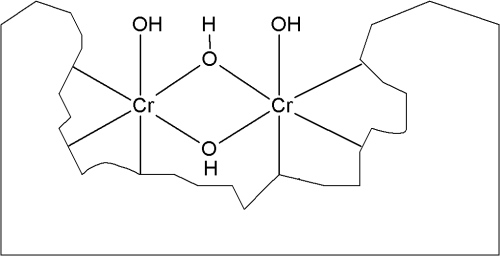
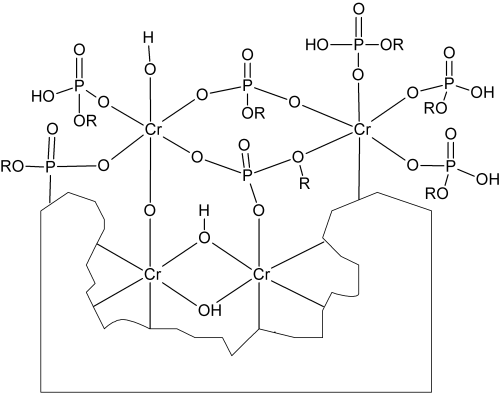
Due to the consumption of oxonium ions, a pH-gradient toward more alkaline pH next to the zinciferous surface develops. In this zone of elevated pH the generated trivalent chromium compounds hydrolyze, thereby generating the respective µ-oxo-bridged and µ-hydroxo-bridged polynuclear trivalent chromium complexes.6 Hexavalent chromium compounds from the solution are adsorbed on the surface of these polynuclear chromium-complex layers (Fig. 2) making up the chromate conversion coating.7
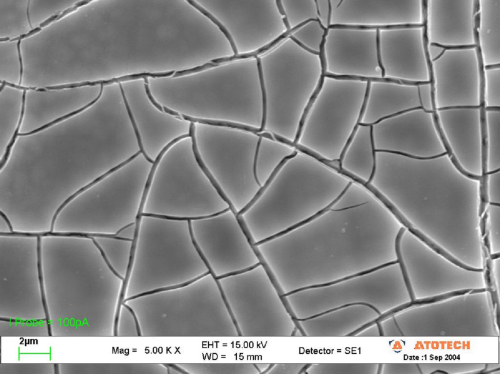
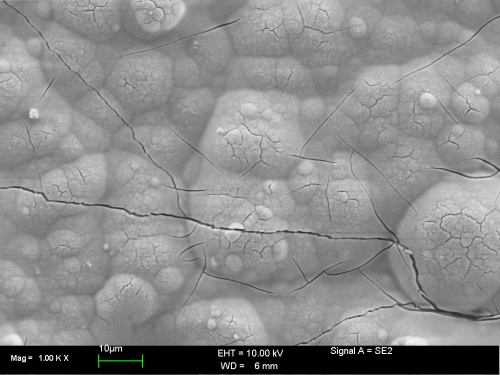
Investigation of the surface of a standard hexavalent chromate on zinc by means of SEM shows the typical fissured, “dry river bed-like” surface of the conversion layer (Fig. 3).
Black chromate conversion coatings can be categorized into zinc and zinc alloy processes. Black chromate conversion coatings on non-alloy zinc use silver evenly spread in the conversion layer as a black pigment.
Yellow chromates on zinc usually show white corrosion products after 240–500 h in neutral salt spray testing according to ISO 9227.8 The corrosion protection of black chromates is found to be noticeably reduced compared to that of yellow chromates with a similar conversion layer thickness. This decreased corrosion resistance can be attributed to silver particles being abundantly present in the chromate layer and also in contact with the zinc surface. Consequently, the zinc in contact with the noble metal is forced to corrode by means of contact corrosion.
The other category of black chromates includes those being applied on zinc alloys like zinc–nickel, zinc–cobalt, or zinc–iron. The black pigment in the chromate layers on these alloys consists of iron, nickel, or cobalt, and their respective oxides, produced on dissolution of some zinc in the acidic process solution. Although these metals are nobler than zinc, the overall corrosion protection of the black chromate conversion coatings achieved on these base metals is much better. Hexavalent chromium-based post-dip solutions were commonly used on such chromates, giving about 240–360 h to white corrosion in neutral salt spray testing according to ISO 9227.
Passivate Conversion Coatings
Layer growth in hexavalent chromate conversion coatings depends on the oxidizing effect of chromates on zinc, consuming acidity and increasing the pH on the zinc/solution interface.
In trivalent passivates, an alternative oxidant taking the role of the chromate is needed. The proper choice of oxidizing agents is crucial for the passivate’s performance. A common, simple example being part of oxidant mixtures in passivates is nitrate. Zinc reduces nitrate according to the formulas given (Dikinis V, et al. Trans Inst Metal Finishing 2004;82[3–4]:98) (see Equation 2).
All these processes consume oxonium ions and, therefore, contribute to higher pH on the zinc surface compared with the bulk solution. Except for the kinetic properties of the individual reduction reactions, ligand exchange on the trivalent chromium ion plays a crucial role in passivate as well as in chromate layer buildup. Lastly, the soluble trivalent chromium compounds in passivates hydrolyze and build up a similar but usually thinner conversion layer on the zinc surface.
These passivate layers, not bearing adsorbed hexavalent chromium on the surface, sometimes lead to inferior corrosion protection results compared to chromates.
Thick-film passivates can be used to generate transparent, iridescent layers with thicknesses of about 300 nm.9 The corrosion protection provided by these layers is similar to that of yellow chromates, although currently not achieved with black passivates.
Generating black finishes using trivalent chromium passivates is similar, in general, to black hexavalent chromates. On zinc, the black pigment may be generated from other transition metals from the process solution. Zinc–nickel or zinc–iron surfaces are usually rendered black by etching the surface, dissolving zinc, and leaving an iron- or nickel-based black pigment within the layer. Although a trivalent chromium conversion coating like the one previously described is also generated simultaneously with these black pigments, the layer’s growth is commonly found to be limited, resulting in inferior corrosion protection results compared with black chromates. Without any further treatment, black trivalent passivates generally protect the respective zinc and zinc–alloy layers only for 24–48 h in neutral salt spray testing (ISO 9227). New developments in pigmenting a chromium conversion coating without using noble metals (with respect to zinc) at Atotech actually enable more stable results of 48 h to white corrosion. However, the current specifications of the automotive industry for unsealed black passivates require higher performance, which, to date, cannot be met without additional post-treatment.
Applying sealers that add an efficient layer to enhance corrosion protection and decorative appearance is precluded from some applications. Alternatively, virtually extending the conversion layer by adding chromium from a trivalent chomium-based solution to the conversion layer is an option.
Consequently, with hexavalent chromium post-dip solutions in mind, the step to post-dip solutions resting on trivalent chromium is obvious.
Trivalent Chromium Approaches in Post-dips
Finding the Proper Coordination Environment for Trivalent Chromium
Choosing the right coordination sphere for hexavalent chromium was not too difficult. The choice of ligands suitable for industrial use is almost completely limited to the oxygen moiety known from chromium trioxide (CrO3), dichromates (Cr2O72–), or chromates (CrO42–).
Upon switching from the 3d0 ion hexavalent chromium to the 3d3 ion trivalent chromium, reactivity and toxicity changes completely. Contrary to hexavalent chromium, the trivalent chromium ion bears exceptionally slow reaction rates with regard to ligand exchange. Also, contrary to hexavalent chromium, the trivalent ion does not act as an oxidizing agent in conversion coating process baths.
The primary criteria for evaluating appropriate ligands are the decorative (aspect) and functional (corrosion protection) properties of the coating.
Keeping the objective in mind that the final composition of the post-dip’s layer should only bear components that are commonly found in passivate layers limits the range of eligible compounds. The formulation of the post-dip solution was adjusted until the best combination was found that achieved satisfactory appearance and corrosion protection performance. By fine adjusting an integrated additive system, the deposition of the post-dip could be attuned towards a highly uniform dispersal of the deposit while imparting a homogenous gloss with a minimized tendency to form drop marks (right panel in Fig. 5).
With the major component of the final composition being trivalent chromium in a carefully adjusted coordination moiety, the surface layer from post-dip treatment does not bear any non-passivate like components.
Variations on the application parameters within reasonable limits around a set of initial figures were conducted in order to establish the final application parameters on black passivated zinc–nickel (Tridur ZnNi H1). Temperature, pH, make-up concentration, and dwell time, as well as drying temperature, have been changed individually, keeping the remaining parameters constant. Application at pH 5.5 (45°C) in a 20% v/v solution has been elaborated to be the optimum set of parameters for the application on Tridur ZnNi H1. Dwell-time variations showed no visible difference within 10–40 s. Drying temperature has been shown to be best between 70–90°C, for 10 min. The results were evaluated for both their decorative aspects and corrosion protection properties.
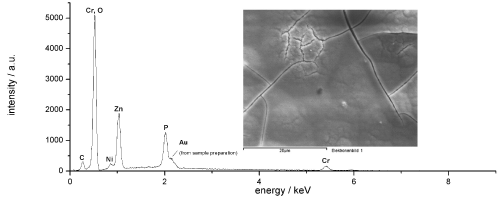
A semi-quantitative EDX analysis of the surface of black passivated zinc–nickel shows only elements, which are typically also found on zinciferous surfaces with trivalent chromium conversion coatings applied (Fig. 6). The deposit from the final formulation adds to the passivate layer. Upon application, the newly developed post-dip acts like a second conversion coating on the conversion layer.
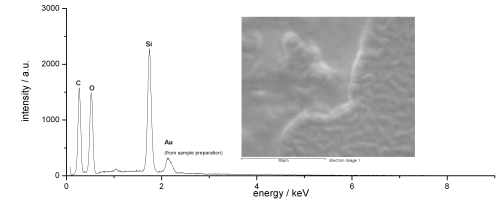
For comparison, the EDX spectrum from black passivated zinc-nickel with an organic polymer/silicate-based sealer (Corrosil Plus 501) applied is shown in Figure 7. Obviously, the layer’s composition is completely different from that of the passivate or the passivate with post-dip system.
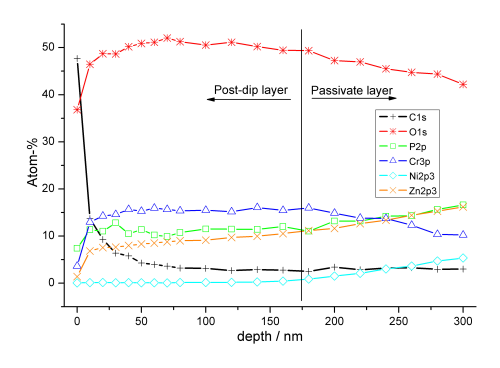
The conversion coating–like composition is confirmed by an XPS depth profile, recorded on a sample of Tridur ZnNi H1 with Tridur Finish 300 (20% v/v) applied (Fig. 8).
Significant carbon concentrations are only found on the surface, likely due to adsorption of CO² from the air or surface contaminations. Within about 10 nm, the carbon concentration falls to a very low level, not changing significantly with increasing sputter depth. The composition of the post-dip layer and the passivate’s conversion coating appear almost identical. A change in nickel concentration indicates a diffuse transition between the more post-dip-like and the more passivate-like layers. Therefore, the post-dip contributes to an increase in thickness of about 0.2 µm in this application.
However, the lack of sharp transitions is also due to the fact that the post-dip penetrates deeply into the passivate layer, effectively filling up micro cracks. Both the passivate layer and the post-dip layer bear reactive sites with regard to coordination chemistry. During the deposition at elevated temperature, and especially in the subsequent hot-air drying process, the trivalent chromium present in the passivate layer reacts with the post-dip solution’s components, finally building up the enhanced conversion coating. Figure 9 shows a structural proposal for this layer’s composition.
Polynuclear trivalent chromium complexes bearing µ-phosphato bridges are described in literature10–12 and they most likely contribute to the post-dip layer’s composition. Due to the very similar composition of Tridur Finish 300 layers and passivate layers, it is very difficult to find some contrast between both layers by means of SEM imaging. However, Figure 10 shows an SEM image of a FIB cross section through a sample with Tridur Finish 300 applied to black passivated zinc–nickel (14% nickel). The image reveals a thickness of 100–200 nm for the passivate and the post-dip layer.
Layer Morphology
The morphology of the post-dip layer was investigated using different concentrations of Tridur Finish 300 applied to a black passivated (Tridur ZnNi H1) zinc–nickel alloy surface. The morphology of the deposit in dependence of the concentration of the post-dip bath was studied by means of SEM micrographs on samples of black passivated zinc–nickel (Figs. 11–14).
The post-dip caulks the micro cracks of the black passivated zinc–nickel surface. The post-dip layer’s appearance itself resembles that observed with a hexavalent black chromate on zinc–nickel with regard to the mud-crack-like surface observed. Above 200 ml/l the post-dip layer’s cracks become larger in size (Fig. 15). This means that with excessive concentrations a lesser extent of the surface may be covered by the post-dip layer. No significant advantage concerning neither the aspect nor the corrosion protection could be determined with higher concentrations.
Corrosion-protection Properties
Corrosion-protection properties were investigated with different concentrations of the post-dip solutions (Tridur Finish 300) applied to black passivated zinc–nickel. It was found that a high level of corrosion protection was already established with 50 ml/l of Tridur Finish 300, not increasing significantly with higher concentrations (100–300 ml/l). However, the aspect of the parts finished was found to be best at 200 ml/l (20% v/v). Evaluation on black passivated zinc–iron (Tridur ZnFe H1) produced similar results. On black passivated zinc (Tridur Zn H1), 100 ml/l was found to be a suitable concentration.
With regard to the decorative aspect of the finished surfaces as well as their corrosion-protection properties by means of neutral salt spray testing, the application parameters shown in Table 1 have been proven in practice for application on some black passivates.
Tridur Finish 300 can be applied in both rack and barrel applications. The bath parameters are the same for both methods and depend only on the composition of the underlying conversion coating.
Tridur Finish 300 is no substitute for sealers in general. Usually the corrosion protection that can be expected from a chromium-based post-dip can be classified as slightly below that of a film-building sealer based on polymer dispersions or solutions (e.g., Corrosil Plus 501).
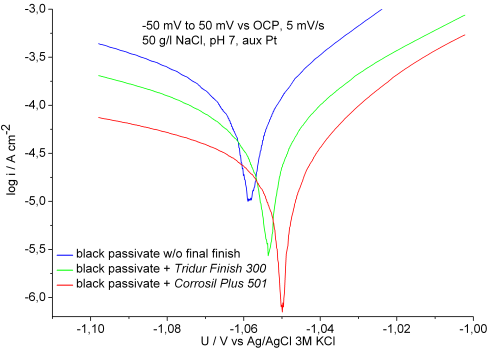
The corrosion behavior was analyzed by recording polarization curves ±50 mV around the open circuit potential in a three-electrode set-up, including a platinum counter electrode, a Ag/AgCl (3M KCl) reference electrode, and the sample as the working electrode. The samples were immersed in aerated solutions of 50 g/l sodium chloride adjusted to pH 7. After 3 min of equilibrium time the open circuit potentials (ocp) were measured and the sample was then polarized from –50 to 50 mV vs. ocp at a sweep rate of 5 mV/s. The data were then plotted on a graph (Fig. 12). The results of the Tafel analysis of the data are summarized in Table 2.
The registered corrosion currents correlate with corrosion rates. The surface with only the passivate and no post-treatment applied showed the highest corrosion rates. Reduced corrosion rates were observed on the surface with the post-dip applied to the black passivate, and even lower corrosion rates were found with the surface having the polymer-based sealer applied. Also, the sealed surface behaves in an electrochemical manner that is slightly nobler than the post-dipped surface, which itself appears nobler than the passivate surface.
This principal sequence in corrosion protection is confirmed by neutral salt spray testing on samples with Tridur ZnNi H1 with Tridur Finish 300 according to DIN EN ISO 9227 (Table 3). Fig. 17 shows three steel panels plated with a Zn/Ni-alloy (14% Ni, 8 µm), black passivated with Tridur ZnNi H1 and with Tridur Finish 300 applied as the final finish after 1,008 h in neutral salt spray testing (DIN EN ISO 9227). Only a small amount of non-voluminous zinc corrosion product formed on the rinsed and dried panels.
Torque and Tension Properties
The friction properties of the new surface were evaluated on M10×50 (thread pitch 1.50) hex head bolts of property class 10.9. The bolts were plated with 8–10 µm of zinc (Protolux 3000) as well as with zinc–nickel (14% Ni, Reflectalloy ZNA) and respectively passivated with a black zinc (Tridur Zn H1) or black zinc–nickel passivate (Tridur ZnNi H1). Tridur Finish 300 (10% v/v for zinc and 20% v/v for Zn/Ni) was applied as the final finish after the passivate treatments. Twelve samples (Zn), respectively 20 bolts (Zn/Ni), were tested on a Schatz Analyse 5413-4504 testing machine at a tightening speed of 30 min–1 according to DIN EN ISO 16047. The results are summarized in Table 4. Higher friction figures have been determined for the zinc–nickel surface compared with the zinc surface. With both surfaces the friction behavior is essentially the same as that found with hexavalent chromium–based conversion coatings (e.g., black or yellow chromates) without any sealer or lubricant applied.
Conclusions
The development of a trivalent chromium–based post-dip solution has been demonstrated and its properties investigated. The post-dip solution does not act like a sealer but reinforces the trivalent chromium–based conversion coating. A µ-phosphate-bridged trivalent chromium complex structure, bearing a similar constitution as that of the passivate layer, has been proposed.
In the course of the development of this additional step of substituting hexavalent with trivalent chromium, several efforts were necessary to adjust the formulation to achieve both the requirements for decorative appearance as well as those of corrosion protection. The objective was to develop a post-treatment process that acts as a second conversion coating and, therefore, can also easily be applied in normal plating equipment. This was successfully achieved with an elaborate new additive system.
This system governs the deposition process in the background without significantly contributing to the layer’s composition. The corrosion-protection properties of surfaces with Tridur Finish 300 applied are found to be excellent but slightly lower than those of surfaces treated with film-building, polymer-based sealers. The tribological properties of the Tridur Finish 300-treated surfaces were essentially the same as those from hexavalent chromates. Although developed with black passivates in mind, the new Tridur Finish 300 final finish process can be applied to any trivalent chromium–based conversion coating in both rack and barrel applications.
While satisfying the high decorative demands issued when switching to trivalent conversion coatings, the new process achieves the corrosion-protection demands of the automotive industry, even with respect to non-sealed black passivated surfaces.
| Base Layer/Passivate | Tridur Finish 300 Post-dip Solution | Corrosion Protection (ISO 9227) | ||
| Concentration | Temperature | pH | ||
| Zinc/Tridur Zn H1 | 100 ml/l | 45°C (40–50°C) |
4.5 (4–5.5) |
>72 H to wc |
| Zinc-iron/Tridur ZnFe H1 | 200 ml/l | 45°C (40–50°C) | 5.5 (5–6.5) | >240 h to wc |
| Zinc-nickel/Tridur ZnNi H1 | 200 ml/l | 45°C (40–50°C) | 5.5 (5–6.5) | >300 h to wc |
| Sample | Ecorr/mV | icorr/µA/cm2 |
| Experimental black zinc passivate without final finish | –1,059 | 89 |
| Experimental black zinc passivate + Tridur Finish 300 | –1,055 | 40 |
| Experimental black passivate + Corrosil Plus 501 | –1,050 | 16 |
| Final Finish Applied | Hours to White Corrosion |
| None/passivate only | 24–48 h |
| Tridur Finish 300 (20% v/v) | 312 h |
| Corrosil Plus 501 BG* | 432 h |
| *Organic polymer/silicate-based sealer. |
| Conversion Coating | F/Nm | T/kN | KM10 | µthread | µhead | µtot |
| Tridur Zn H1 | 120.3 ± 11.3 | 36.1 ± 0.01 | 0.33 ± 0.03 | 0.32 ± 0.04 | 0.22 ± 0.04 | 0.27 ± 0.03 |
| Tridur ZnNi H1 | 150.9 ± 13.7 | 36.1 ± 0.02 | 0.42 ± 0.04 | 0.33 ± 0.07 | 0.35 ± 0.03 | 0.34 ± 0.03 |
Notes
- Wilhelm, E.J., US Patent 2,035,380.
- Johnson, D.M., US Patent 2,559,878.
- Directive 2000/53/EC of the European Parliament and of the Council of 18th of Sept. 2000, on end-of-live-vehicles.
- Directive 2002/95/EG of the European Parliament and of the Council of 27th of Jan. 2003.
- Directive 2002/96/EC of the European Parliament and of the Council of the 27th of Jan. 2003, on waste electrical and electronic equipment.
- Lukaszewski, G.M., Redfern, J.P. Nature 1961;190:805–6.
- Bard, A.J., Frankel, M, Stratmann, M. Encyclopedia of Electrochemistry. Vol. 4. Weinheim: Wiley-VCH, 2003.
- Jelinek, T.W. Galvanisches Verzinken. Saulgau: Eugen G. Leuze Verlag, 1982.
- Sonntag, B, Vogel, R. Galvanotechnik 2003;10:2408–13.
- Redfern, J.P., Salmon, J.E. J Chem Soc 1961;291.
- Springborg, J. Acta Chem Scand 1992;46:906–8.
- Haromy, T.P., Linck, C.F., Cleland, W.W., Sundaralingam, M. Acta Cryst 1990;C46:951–7.
Bio
Dr. Björn Dingwerth leads the R&D team for corrosion-resistant coatings at Atotech’s Berlin headquarters. He has seven years of experience in the metal finishing industry and primarily works on zinc and zinc-alloy deposition, as well as their post-treatment processes. Dingwerth received his Ph.D. in chemistry from Heinrich-Heine-University in Düsseldorf, Germany.





