1. INTRODUCTION Phosphating is the most widely used pretreatment process for the surface finishing of metals. The corrosion protection performance of such finished products is determined mainly by the quality of the phosphating coating. In today’s metal finishing industry, the multi-stage phosphating technologies are commonly used [1]. Normally, the metal surface is degreased, rinsed, phosphated, possibly sealed (with hot water or carcinogenic chromates), dried, and finally painted [2,3]. However, the multi-stage phosphating technologies are error-prone, costly and produce wastes including organic solvents, heavy metals, and other toxic and deleterious materials [4]. The phosphating coatings thus obtained usually bear a porosity of 0.5%-1.5% of the total substrate surface area [2,5,6]. A subsequent rinsing and sealing procedure, which traditionally utilizes highly toxic hexavalent chromate (Cr6+) compounds becomes very critical to the corrosion protective performance of the pre-treated surface [2,7]. If these pores are not quickly sealed or recoated, they begin to deteriorate shortly the phosphating coatings after application. Therefore, it would be advantageous to develop a novel surface treatment technology that provides complete coverage of the base metal without the need for a finishing step.
The in-situ phosphating coating (ISPC), which was developed by the Michael Faraday Laboratory of Norther Illinois University in the 1990s, is just such a new surface treatment technology [1]. ISPC is prepared by predispersing an optimum amount of in-situ phosphating reagent (ISPR) in the paint system to form a stable and compatible coating formulation [8]. When applied on a metal substrate, it allows the phosphating process to take place in-situ via the reaction of ISPR with the metal surface. Simultaneously, ISPR reacts with the polymers, forming strong covalent bonds of P-O-C (phosphorus-oxygen-carbon), linking the metal phosphate thin film and the polymer resin matrix. These “simultaneous” reactions provide metal substrates with a corrosion protective barrier without the need for a chromate or primer finishing step. It might also be possible to eventually eliminate the pretreatment and/or post-treatment steps and to obtain a coating providing a better adhesion and corrosion inhibition to the substrate [8,9,10].
Chhiu-Tsu Lin[7] applied ISPCs to three types of commercial paints (solvent-borne high-solids, water-reducible and VOC-free latex) on bare and pre-treated cold-rolled steel and 2024 aluminum test panels. The results showed that the protective performance (coating adhesion and corrosion inhibition) of ISPCs is superior over that of the multi-step phosphating coating practice. Mary C.Whitten et al [10] investigated the effect of catalyst and pigment on polyester-melamine in-situ phosphating coating on a cold-rolled steel system. The EIS data showed that a pigmentless polyester-melamine coating catalyzed with p-toluenesulfonic acid (p-TSA) shows high impedance (~1010 Ω·cm2) at low frequency (0.1 Hz). The corresponding electrical equivalent circuits showed that the in-situ phosphating coating (ISPC) applied to both bare and pretreated steel panels provided superior corrosion protection. Heather Neuder et al[11] selected functionalized arylphosphoric acid, functionalized arylphosphonic acid as ISPR and epoxy/polyamide mixture as a primer in order to generate the in-situ phosphating coating on aluminum alloy. EIS measurements demonstrated that the ISPC primer on untreated 2024-T3 Al test panels showed 10–100 times more resistance than the control primer on 2024-T3 Bare/Alodine 1200. The chemistry of the simultaneous reaction of ISPRs catalyzed the curing of the paint film. The FTIR reflectance spectrum indicated that the metal-phosphate layer was formed at the interface of paint and aluminum substrate. The development of ISPC relies critically on the selection of an effective ISPR, which can be predispersed in the paint system to form stable and compatible coatings when applied and cured on the metal substrate.
For the present experiment, an alkyd ready-mixed paint was applied to untreated AA6061 surface to form a paint coating. Phenylphosphonic acid (PPA) was selected as ISPR. Electrochemical measurement and salt water immersion (SWI) test are used to evaluate the corrosion resistance of coating. 2. EXPERIMENTAL 2.1 Paint system An alkyd ready mixed paint system was prepared using the following components in percentages by weight: C-03 modified alkyd resin (38.6%), CJ-075(cross-linking agent, 2.8% ), CG-09X (an anti-settling agent, 0.7%), W-791 (silicon dioxide, 0.2%), T50-80(titanium dioxide, 42.7%), and solvents [n-butanol (2.7%), 2-butoxyethanol (4.5%), methyl ethyl ketone (1.3%), and xylenes (6.5%)]. The paint formulation was obtained from Daihe Paint Co., Ltd, and made without any acid catalyst added. Phenylphosphonic acid (PPA), which was supplied by Yi Ren Chemical Technology Co., Ltd., was employed as ISPR. The ISPR was dispersed directly into the paint formulation by a high speed mixer. All chemicals are used as received.
Next, the paint solution was separated into different containers and had PPA added in various percentages by weight of the paint formulation (i.e., 1% PPA was made by mixing 100g of the paint solution with 1g of PPA). 2.2 Treatments of samples The commercial AA6061-T6 alloy panels used as substrates were purchased from Ming-Hua Metal Materials Co., Ltd. Guangdong. The chemical composition of AA6061 is given in Table 1.
The rectangle panels (50 mm×25 mm×1 mm) of AA6061 were mechanically polished to give a mirror-finished surface and rinsed with water and ethanol, then blown dry with hot air. After the Al panels were spray painted using nitrogen as the propellant, the painted panels were flashed for 30 min, and then were cured at different temperature and time. 2.3 Test techniques By using a three-electrode cell composed of a saturated calomel electrode (SCE) used for the reference electrode, a platinum electrode used for the counter electrode, and the painted Al panels (1cm×1cm of exposed area) used for the working electrode, the potentiodynamic polarization measurements of the panels were conducted in 3.5% NaCl solution in equilibrium with air at 25? using CHI700B electrochemical workstation (CHENHUA Instrument Co., Ltd. Shanghai) with corresponding software for analysis. In potentiodynamic polarization experiment, the potential range was from -2.4SCE to 0.2 VSCE with a scan rate of 0.5mV/s. All the painted Al panels were preimmersed in the electrolyte for 12h before data acquisition in order to achieve a steady state.
Salt water immersion (SWI) tests were carried out by submerging the painted Al panels in a 3.5% NaCl solution for 21 days. For fully painted panels, before immersion tests, two crossed lines on the surface of panels were scored to the metal substrate using a razor to aid in the corrosion process according to ISO 2409 standard [12].
The anticorrosion performance was estimated by the width of the enlarged rust along the scribe after the SWI test.
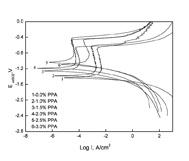
On average, three replicate panels were tested for each condition in every test. 3. RESULTS AND DISCUSSION 3.1 Potentiodynamic polarization curves Figure 1 shows the polarization curves of the ISPC formed on AA6061 panels in the paint solution containing different contents of PPA in 3.5% NaCl solution. Before the electrochemical experiments, the painted panels were cured 8h at 50?. Corresponding fitting results are presented in Table 2. The corrosion current density, Icorr(which increases with corrosion rate), was estimated by extrapolating the cathodic branch according to a Tafel analysis.
According to Figure 1 and Table 2, we can know the trend of the Ecorr and Icorr values with increase in PPA content in the alkyd ready-mixed paint solution. Adding small amounts of PPA in alkyd ready-mixed paint solutions shifted the corrosion potentials Ecorr to more noble. Compared with that of the paint coating formed in paint solution without PPA (Curve 1), the Ecorr value of the ISPC formed in the paint solution with 1.0%, 1.5%, 2.0%, 2.5%, 3.0% PPA (Curve2-6) increases by about 40, 171, 241, 394, 339 mV, respectively. At the same time, the potentiodynamic polarization curves of the curve 2-6 have shifted left, that is, transferred to low corrosion current density. Compared with that of the curve 1, the Icorr values of the curve 3-6 decline one order of magnitude, and the Icorr value of curve 2 drops by 63%. The decrease of corrosion current density indicates the reduction of the dissolution rate of aluminum alloy.
According to the literature[7,10], PPA reacts in-situ with the aluminum surface to produce a metal phosphate layer at the aluminum and alkyd ready mixed paint coating interface, and simultaneously forms covalent P–O–C linkages, which can seal pores that may be present at the metal phosphate layer, with the alkyd resin. Sealing the pores in metal phosphate layer by covalent P–O–C linkages can effectively hinder the transfer of corrosion products between aluminum alloy surface and corrosive media and inhibit the corrosion reaction of AA6061 panels. Therefore, the alkyd ready-mixed paint coating containing PPA as in-situ phosphatizing reagent (ISPR) can improve the corrosion resistance of AA6061 panels in 3.5% NaCl solution.
It should be noticed that, when the content of PPA in alkyd ready-mixed paint solution was 3%, the Icorr of the ISPC (the curve 6 shown in Figure 1 and Table 2) formed on the AA6061 panel somewhat increased. It may be because that the excess PPA caused the ISPC to be excessively acidic [13]. The excess acid could make the paint coating become more hydrophilic, thereby resulting in accessible to electrolytes in the presence of salt solutions and debasing the protective performance of the paint coating [14].
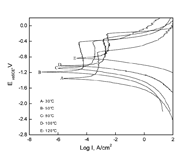
The thermal curing temperature and time will affect the protective performance of the paint coating [15]. Figure 2 shows the potentiodynamic polarization curves of the 2% PPA-catalyzed paint coating which was cured at different thermal curing temperature for 8h. Corresponding fitting results are presented in Table 3.
At present, the detailed chemical mechanism that occurs during the entire curing process of the paint coating is understood. However, three categories of polymerization reactions have been proposed [16]. They are (1) reactions that form new covalent bonds and increase cross-link densities, (2) pairs of reversible reactions in which covalent bonds are broken, but new ones formed, and (3) reactions which break bonds irreversibly. Figure 2 and Table 3 show that the corrosion current density of the 2% PPA- catalyzed paint coating (the curve B shown in Figure 2 and Table 3) cured at 50? is the smallest. It implied that PPA catalyzed polymerization of the paint coating, resulting in forming new covalent bonds and increasing cross-link densities of the paint coating during the entire curing process at 50?. The coating with high cross-link density is considered a protective barrier [17]. When the curing temperature was low (for example, curve A at 30?), the polymerization of the paint coating was not completely and the cross-link density of the paint coating was lower[14], causing the corrosion resistance of the paint coating to decrease. At this time, the paint coating was observed to be soft and undercured. However, the high curing temperature will also reduce the cross-link density of the paint coating. It was found in the experiment that the paint coating cured at 120? (curve E) became more brittleness and slightly darker in color, there were some cracks and slight defects, which caused the paint coating resistance to decrease on the paint coating surface.
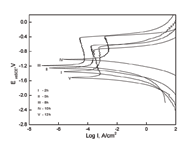
Figure 3 shows the potentiodynamic polarization curves of the 2% PPA-catalyzed PAINT COATING which was cured at different thermal curing time at 50?. Corresponding fitting results are presented in Table 4.
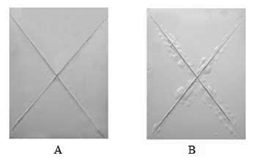
The resulting data from Figure 4 and Table 3 showed that short or long curing time was not beneficial to the polymerization of the paint coating. If curing time is too short, the polymerization reaction is not complete. It will result in a low cross-link density. If curing time is too long, the paint will become brittle and crack [17]. 3.2 Saltwater Immersion Test The salt water immersion (SWI) test is a very good qualitative test to determine the paint’s corrosion-inhibition performance. The saltwater immersion results are shown in Figure 4.
The saltwater immersion studies showed that, after the painted panels had been submerged in a 3.5 % NaCl solution for 21 days, the paint coating that was catalyzed with 2% PPA cured at 50? for 8h produced good protective performance; no blisters were formed in areas of the panel that were exposed to the salt solution (shown in Figure 4, A). However, there were small clumps of blisters on the paint coating that was not catalyzed with PPA (shown in Figure 4, B). This is attributed to PPA’s ability to phosphatize the metal substrate in-situ. The acidic nature of the PPA is able to catalyze the thermal curing of the paint coating, while the phosphating functionality of the PPA can migrate to the surface of the substrate, produce a metal phosphate layer, and chemically bond to the metal, sealing up pores which generally allow electrolytes through to corrode the metal. 4. CONCLUSIONS The results of potentiodynamic polarization curves and salt water immersion (SWI) test reveal that phenyl phosphonic acid (PPA) as an in-situ phosphating reagent (ISPR) can effectively improve the corrosion resistance of alkyd ready-mixed paint coating formed on AA6061 in the 3.5% NaCl solution. However, the corrosion resistance of the in-situ phosphating coating (ISPC) is relevant with the contents of phenyl phosphonic acid (PPA) in alkyd ready mixed paint coating. In the present study, the optimum content of phenyl phosphonic acid (PPA) in alkyd ready mixed paint coating cured at cured at 50? for 8h is 2%.
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
| 0.4–0.8 | 0.70 | 0.15–0.4 | 0.15 | 0.8–1.2 | 0.04–0.35 | 0.25 | 0.15 | Balance |
| No | Content of PPA (%) | Ecorr (mV) | Icorr (mA/cm2) |
|
1 2 3 4 5 6 |
0.0 1.0 1.5 2.0 2.5 3.0 |
-1428 -1388 -1257 -1187 -1034 -1089 | 0.6601 0.2463 0.02762 0.01282 0.02313 0.03825 |
| No | thermal curing temperature (oC) | Ecorr (mV) | Ecorr (mV) |
| A B C D E | 30 50 80 100 120 | -1356 -1187 -1089 -1040 -840 | 0.04503 0.01282 0.01457 0.1403 0.4196 |
| No | Curing time (h) | Ecorr (mV) | Ecorr (mV) |
| I II III IV V | 2 5 8 10 12 | -1353 -1261 -1187 -1020 -1515 | 0.03399 0.02255 0.01282 0.1432 0.1495 |
ACKNOWLEDGEMENTS The authors gratefully would like to thank Tang Yaoming for helpful discussions and the assistance in the electrochemical measurements REFERENCES
- Yu T, Lin C. T, Ind. Eng. Chem. Res. 36 (1997) 368.
- Freeman D. B, Phosphating and Metal Pretreatment; Industrial Press: New York, 1986.
- Spadafora S. J, Hegedus C.R, Hirst D.J, Eng A.T, Mod. Paint Coat. 80(1990)36.
- Paint Waste Reduction and Disposal Options; Hazardous Waste Research and Information Center: Champaign, IL, 1992; Vol. I (HWRIC RR-060) and II (HWRIC TR-008).
- Rausch W, The Phosphating of Metal; ASM International: Metal Park, and Finishing Publications Ltd.: Teddington, Middlesex, England, 1990.
- Narayanan T.S, M Subbaiyan, Metal Finishing. 91 (1993) 89.
- Lin C. T, Prog.Org.Coat. 42(2001)226.
- Lin C. T, Lin P, Hsiao M.W, Meldrum D.A, Ind. Eng. Chem. Res. 31(1992)424.
- Yu T, Li L, Lin C.T, J. Phys. Chem. 99(1995)7613.
- Whitten M.C., Chuang Y. Y, Lin C. T, Ind. Eng. Chem. Res. 41(2002)5232.
- Neuder H, Sizemore C, Kolody M, Chiang R, Lin C. T,. Prog.Org.Coat. 47(2003) 225.
- ISO Standards Handbook: Paints and varnishes: Volume 1. General test methods: Part 1, ISO2409 code, 2002, International Organization for Standardization.
- Brooman E W, Metal Finishing. 100 (2002)48.
- Li L, Lin C.T. Ind. Eng. Chem. Res. 33 (1994)3241.
- Lin C. T, Lin P, Hsiao M. W, Meldrum D.A, Martin F. L, Ind. Eng. Chem. Res. 31 (1992)424.
- Gan S, Solimeno R.D, Jones F. N, Hill L.W, Proceedings of Sixteenth Water-Borne and Higher-Solid Coatings Symposium, New Orleans, LA; The University of Southern Mississippi: Mattiesburg, MS, 1989.
- Whitten M C, Lin C.T. Ind. Eng. Chem. Res. 38 (1999)3903.
BIO Zhang Ming-ming, graduated from the College of Chemistry & Environmental Science, Henan Normal University in 2008 and now work in Technical Institute of Physics and Chemistry,Chinese Academy of Sciences.
* Corresponding author. Address: Technical Institute of Physics and Chemistry,Chinese Academy of Sciences, Beijing, China,100190. E-mail: mingmingzhang64@yahoo.com.cn






