A solution to this conundrum is to use mixtures of solvents. The basic idea is simple and has two parts:
- That the physical, chemical, and cleaning properties of the mixture will be determined from those of its components. In other words, mixtures are physical combinations not products of chemical reaction.
- That certain mixtures of solvents exhibit the distillation properties of a single solvent, and can be managed that way. In other words, the mixture doesn’t separate into its components when boiled in a vapor degreaser.
Mixtures
The reason for using mixtures for solvent cleaning work is flexibility. As chemicals used in metal finishing become more capable, the chemicals (aqueous or solvent) used to clean them must also improve. We’re pleased to learn when a coolant product is enhanced with a better corrosion inhibitor, lubricant, or biocide. But, what about the cleaning chemical (solvent)? Does it need to be enhanced as well?
That the cleaning chemical might need augmentation to deal with additional or different soil components is the reason for flexibility.
The need for continuous improvement means that others in our organization will find new ways to get parts dirty. So those having to clean the new dirt will need new capability.
New equipment, new chemicals, and new procedures all add capability. But they add cost and complexity too. The most commonly taken action is the simplest one—to add another component to the cleaning chemicals that compensates for the component added to the soil being cleaned. This is practical continuous improvement!
Changing the cleaning solvent by addition of a second solvent means the cleaning process won’t perform as it did. The reason is enrichment.
Enrichment
This doesn’t have anything to do with investments! Enrichment means simply that the most volatile component in a mixture of boiling solvents is found at a higher concentration in the vapor than in the boiling liquid. Enrichment is a phenomenon associated with any mixture of liquids where one of the components is more volatile than any or all of the other components.
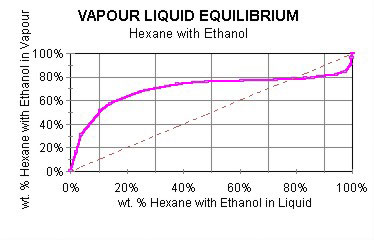
Said another way, the vapor phase is enriched in the most volatile component. If the vapor and liquid compositions are identical, there is no enrichment. This is shown in Figure 1.
Enrichment is fatal for vapor degreasing operation. If the composition of the boiling bath (or the condensing vapor) changes, the quality of the cleaning performance will change.
Experience with a Vapor Degreaser
Suppose you operate a conventional open-top vapor degreaser with a solvent mixture. Your unit is assumed to be operating within U.S. required limits for environmental control (fancy that!), and is using a solvent mixture that is a 50/50 (by weight) mixture of hexane and ethyl alcohol.
Hexane is the most volatile component because its boiling point is somewhat lower than that of ethanol (68.7°C vs 78.3°C).
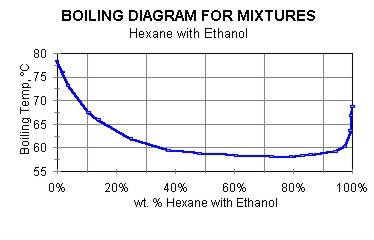
If the mixture is boiled, hexane will be the component preferentially lost. In this situation, the mixture will become richer in ethanol. Consequently, the boiling temperature will also change. The boiling point diagram for hexane and ethanol is shown in Figure 2.
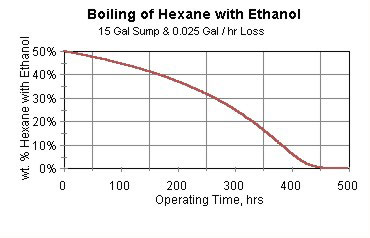
When the vapor degreaser is started, dirty parts can be cleaned. A small amount of vaporized mixture solvent is emitted (while being compliant with the U.S. EPA’s NESHAP), and the boiling sump becomes richer in ethanol and leaner in hexane.
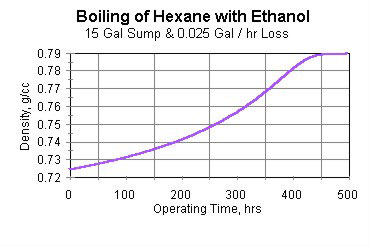
In fact, after about three weeks of operation, all the hexane has been lost due to evaporation—without violation of the U.S. EPA’s NESHAP. At this point the boiling sump is pure ethanol. This is shown in Figure 3.
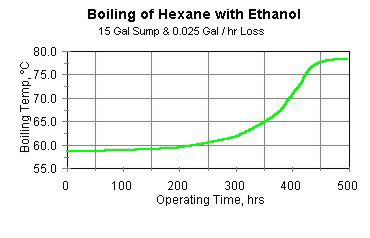
An alert operating crew might have noticed that the sump level and boiling temperature of the degreaser had changed. If the crew had taken a sample of the cleaning solvent during a shutdown, they might have noticed that its density had changed. These changes are shown in Figures 4 (boiling temperature) and 5 (solvent density).
Cleaning Performance of a Vapor Degreaser
These two components have some similar physical properties. Naturally, because the solvents have different structures, solvency properties (Hansen Solubility Parameters [HSP]) are not similar. That’s why a two-component mixture was used—to provide solvency for two different soil components.
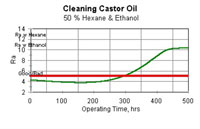
Change of composition as shown in Figure 3 means that there will be a change in cleaning performance, because the HSP values of the solvent blend will have changed to those of pure ethanol (not that pure ethanol is bad). Calculations showing the change in cleaning performance of an assumed soil against an assumed cleanliness standard are shown in Figure 6.
Consequences and Lesson
If you use solvent blends in an open-top vapor degreaser this will happen to you—unless you and your crew monitor its condition on a continuing basis and correct the mixture composition by adding the solvent component which is most volatile.
Don’t be swayed by claims of improved performance with solvent blends—if you are considering their use in an open-top vapor degreaser. Just the loss by normal emission of the amount allowed under environmental regulations will totally destroy integrity of your unit in a matter of weeks.
The Solution
There are two possibilities: to change the equipment or to change the solvent(s).
An enclosed vapor degreaser can be an excellent solution. It can allow compliance with environmental regulations in the Los Angeles basin. Emission rates can be almost negligible, hence the situation described in Figure 6 is avoided.
The second solution is to use mixtures whose composition doesn’t change if their vapor is removed from the system. These mixtures are called azeotropes and they will be covered in Part II of this column.
References
- N-propyl bromide was a “fluke.” It was not developed as a cleaning product. An often-repeated story, possibly even true, was that a research chemist (Dr. Burnell Lee) noticed in the spring of 1995 that N-propyl bromide had the same distillation properties as 1,1,1 trichloroethane and was also a pretty good solvent. N-propyl bromide had been manufactured for many years as a way of conveying a bromine atom from those who manufactured raw materials for products containing bromine atoms (fire suppressants, reaction initiators, alkylating agents, biocides, etc.), to those who actually synthesized those products.
- Kenyon, W.G., “Don’t Look for New Solvents,” SMT Magazine, page 16; June 2004
- Many processes, including distillation, are based solely on enrichment. Gasoline is refined from crude oil in this way. Distillation is often used to recover solvents from soils in a distillation column attached to vapor degreasers. The soils are usually much less volatile than the solvent(s).
- For this example, the sump volume is 15 gallons. The sump is 18 inches deep. Top area is 12 in. by 15 in. These values describe a commonly-used small unit.
- In 1995, the U.S. EPA promulgated a National Emission Standard for Hazardous Air Pollutants (NESHAP) that applied to solvent vapor degreasers. The reference is EPA-453/F-94-083. Batch units are limited to 30.7 lb/month of total solvent loss when operating.
- For this example, mixture boiling point is about 58°C. Density of this mixture is about 0.72 g/cc. This mixture was selected for a reason all too common when solvent mixtures are used for cleaning—multiple types of soils, or a single soil with multiple functionality. Hexane boils at 68.7°C and its density is 0.659 g/cc. Values for ethanol are 78.3°C and 0.790 g/cc.
- The disperse, polar, and hydrogen-bonding HSP values for hexane are: 14.9, 0.0, and 0.0, respectively. The same values for ethanol are 15.8, 18.8, and 19.4. The polar and hydrogen-bonding values are naturally different because these solvents were chosen to clean two quite different soils.
- For this example, it is assumed that the cumulative loss of liquid volume in the sump is not recognized—an unfortunately all too common circumstance.
- Hansen Solubility Parameters (HSP) were described in this column in the January 2004 (pages 39–42) and April 2004 (pages 42–59) issues of Metal Finishing.
- Figure 6 shows expected cleaning performance, as characterized by a term called R. The horizontal line is the assumed standard. Values of expected cleaning performance (poor) for the two single solvents are shown in the upper left corner of Figure 6. The soil was assumed to be well-matched to the 50/50 hexane/ethanol mixture.
- There are two types of enclosed vapor degreasers—those sealed against pressure, and those sealed against vacuum. The latter is by far the most common.
Contact the author






