

THE FALLACY OF FLASH POINT RELATIVE TO AEROSOL BLENDS
Many believe that aerosols are safe below their flash points. Serious fires have occurred in the chemical process industries because of that belief.
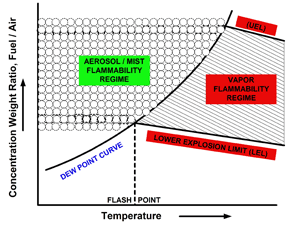
In fact, aerosols of combustible liquids at temperatures well below their flash points can be as ignited as can vapor-air mixtures of flammable liquids. Eichhorn1 made this observation more than a half-century ago, and introduced a conceptual idealized diagram to describe this situation. See Figure 1.
Many are also of the opinion that aerosols composed of organic solvents that do not have a measured flash point cannot be ignited. Such is unfortunately not the case, because flash point is measured in an all-vapor phase, not in the two-phase vapor-liquid mixture that is an aerosol.
Absence of a measured flash point, for a single component or a mixture, means that ignition was not noticed when a spark of specific intensity was applied to organic fuel(s) and air in a specific, and not industrially relevant, apparatus.
In other words, it is not that the mixture can't be ignited, it is that it cannot be ignited in that specific apparatus in the vapor phase. The mixture may well be ignitable in some other test apparatus or in your shop if spark sources aren't eliminated.
In immersion cleaning with organic solvents, flash point determines which electrical classification will apply. In spray cleaning, however, the flash point of the liquid does not determine which electrical classifications will apply.
Use of liquid sprays in air is covered by OSHA (U.S. Occupational Health and Safety Administration), with strong assistance from the NFPA (National Fire Protection Association), in OSHA 1910.123-126, and .107.
A flammable aerosol is defined by the OSHA as "an aerosol which is required to be labeled flammable under the Federal Hazardous Substances Labeling Act (15 U.S.C. 1261). For the purposes of paragraph (d) of this section, such aerosols are considered Class IA liquids."
HIGH FLASH POINT MATERIALS CAN FORM AEROSOLS
Design of heat exchangers in chemical plants requires acceptance of the meaning of the title of this section. These heat exchangers can use high boiling organic liquids which have high flash points as coolants to remove heat. A safety problem is that any leak of these pressurized high-flash liquids from a pipe, a weld, a seal, or a pump will form an aerosol in the same way as does a low flash material propelled from an aerosol can.
To summarize, organic liquids with nearly all values of flash point can form an aerosol by being forced by pressure through an aperture, thereby producing an aerosol. Some can be ignited depending upon their atomic composition, and some cannot for the same reason.
THE MEANING OF EXPLOSIVE LIMITS
A limiting condition about ignitability of aerosols is the vapor phase, which is equivalent to a droplet size of zero. That's the condition in which flammability tests (LEL / UEL) are done (see Figure 1) the vapor phase, and not the two-phase condition of an aerosol.
A solvent blend that has no measured lower explosive limit (LEL), or upper explosive limit (UEL), offers no quantifiable margin of safety when it's emitted as an aerosol spray. Simply because it couldn't be ignited in a standard vapor-phase flammability test apparatus, that does not model the two-phase condition of an aerosol.
So LEL and UEL values don't specifically apply to the two-phase condition of an aerosol. They do speak to the effect of atomic composition on the ignitability of a solvent mixture in the vapor phase, but not in the two-phase condition of an aerosol.
Solvents, or solvent blends, without measured LEL values used as aerosols are probably but not measurably more safe from ignition; and so are somewhat preferred.
PREVENTION OF IGNITION OF AEROSOLS
Many ignore the potential for this outcome because aerosol cans are commonly used, and shop fires with their use are not common. But such fires can and do happen. Fortunately in many cases the experience is less than fatal because the mass of flammable goods in the can (including propellant) is less than one-half pound (12 volumetric ounces capacity is common).
The larger flammability hazard from use of aerosol cans is that a hand-held fire will lead to ignition of some larger quantity of fuel by a person not accustomed to managing this hazard.
There is only one cause of ignition of a can of aerosol cleaner that a ignition source has been generated in the zone where the aerosol has been sprayed, or has been directed by ventilation.
An incomplete list of sources of sparks includes:
- Gas or liquid-fueled open-flame equipment (naked flames),
- Cigarettes and associated matches/lighters (open flames),
- Static electricity from mechanical equipment or motion (which can be dampened by mats as is done in fab shops producing semiconductors though not for that reason),
- Hot processes/hot work (welding by contractors or shrink wrapping),
- Failure of temperature control thermostats on hot work processes, and
- Heat sources (gas, electric, microwaves, radio frequency, thermal fluids).
Tactics for preventing experience of the image of Figure 2, by avoiding and quenching ignition sources, are simple and proven develop, follow, and audit a program of local safety regulations.
Available from for-profit training companies, they are also available at no charge from reputable sources such as U.S. OSHA,NFPA, a local insurance carrier serving the site, and the Internet. The most important tactic is for a responsible leader to audit compliance, over and over and over again.
Not as a substitute for managing elimination of ignition sources, the second (and perhaps more important) priority of action is to manage area ventilation of air.
Aerosol material emitted from a spray can has two general fates. Some (hopefully most) wets the soiled surface, and remains there. The remainder exists above the work area, suspended in the air. Ventilation is necessary to carry away aerosol droplets in that air so they are not inhaled by workers.
The "amount" of ventilation is sized by specifying a velocity of air in whatever area of ductwork is used to carry away aerosol fumes from where spray cleaning work has been done. That velocity must be more than sufficient to maintain solvent aerosols droplets (particles) in suspension in the exhaust air, but not so much as to incur excessive energy loss or produce noise. Larger droplets settle more rapidly (changing by the square of the droplet diameter), requiring higher velocities to suspend them. OSHA ventilation standard 1910.125 (dipping and coating work with organic solvents) is a relevant reference.
HAZARDS OF SOLVENT AEROSOLS TO HUMAN RESPIRATORY SYSTEMS
The hazard presented by an aerosol droplet to the respiratory system firstly depends upon the site within the respiratory system where it is deposited, and secondly upon its inherent toxicity at the point of deposition. And the path to that site is determined by its aerodynamics in the air stream of breath, and aerodynamics are all about droplet size.
Droplets of a respirable aerosol greater than around 30 microns (possibly barely visible to the eye) aren't well entrained in incoming air. It is likely many settle (because their inertia outweighs the buoyant force of the moving breath) outside human bodies, though some are trapped in the nose and mouth on breathing. This is called an inertial separation mechanism.
Droplets sized between around 10 to 30 microns (not visible to the eye) penetrate into the curving torturous path that is the throat (pharynx) but impact on and stick to wet tissue surfaces. So they become deposited in the airways of the head.
Smaller droplets ("thoracic aerosols") can penetrate deeply into the lungs. The myriad of passages within the alveoli (gas exchange region) of the lungs make them a "final" site for inertial separation of droplets by particle size(s). Nearly all droplets above about 1 micron are deposited there sticking onto wet lung tissue.
Solvent in the vapor phase would penetrate to this position as well. But the crucial difference, what makes exposure limits of solvent aerosols be so low, is essentially density of contact.
The mass of liquid solvent held in a droplet of less than 1 micron diameter contains huge numbers of solvent molecules that are all deposited where that droplet becomes trapped on wet lung tissue, while vapor also contains huge numbers of solvent molecules that condense over the more than 50 square meters of wet lung tissue.
The effect of inhaling aerosols is to damage one's lungs by administering a large dose of whatever toxic harm the solvent presents to a myriad of sites where oxygen transfer with the blood is accomplished.
Aerosols thus become an amplifier for application of toxic damage to the lungs the applied dose is exaggerated vs. exposure to solvent vapor. And solvents which are less toxic, and have higher exposure limits, must be treated as if they were more toxic, and have lower exposure limits.
While the exposure limit values promulgated by ACGIH about inhalation toxicity for solvent aerosols are scarce, comparison to the same data for solvent vapors is striking.
TLVs for solvent aerosols are typically two orders of magnitude below those for solvent vapors.
Certainly, details of the imposed harm vary with the specific solvent, and the existing condition of impacted lung tissue. Persons with asthma are not likely to be able to manage inhalation of solvent aerosols.
For these reasons, some prefer aerosols which are not dispensed through expansion of a propellant, and choose those dispensed through hand-pumped sprays. The latter produce relatively huge droplets thereby avoiding the harm described above.
Unfortunately, the solvent cleaning agents described in Table 1 aren't commercially packaged in suitable containers for that method of application as they are too volatile and expensive.
The hazards described above can be overcome, as can most involved with use of solvents. The approach is simple: obtain and use personal protective equipment. An operator applying solvents to surfaces via aerosol delivery should at a minimum wear an N95 hospital breathing mask (and possibly appropriate gloves) both during and after use; at maximum, wear a self-contained respiration system.
The former is shown in Figure 3. Proven specifications are that it will remove more than 95 percent of particles (droplets) whose sizes are above 0.3 microns. Every hospital supply store dispenses them at reasonable cost.
Summary About Aerosol-Dispensed Solvents
The nature of solvent cleaning with aerosols is different than that of solvent cleaning under immersion. A combination of mechanisms is involved surface wetting, solutioning, penetration of networks by diffusion, and hand-applied mechanical force.
One selects a solvent blend for aerosol cleaning to avoid ignition of the solvents once they have evaporated, and to best enable the combination of mechanisms noted above.
And one has to recognize that the hazards of using a solvent blend dispensed from aerosol cans are significantly more threatening to humans than is imposed by using that same blend in immersion cleaning. Fortunately, there is a remedy for that increase of threat to the respiratory system wear a N95 hospital mask.BIO John Durkee is the author of the book Management of Industrial Cleaning Technology and Processes, published by Elsevier (ISBN 0-0804-48887). He is an independent consultant specializing in metal and critical cleaning. You can contact him at PO Box 847, Hunt, TX 78024 or 122 Ridge Road West, Hunt, TX 78024; 830-238-7610; Fax 612-677-3170; or jdurkee@precisioncleaning.com.
REFERENCES
- Eichhorn, J. “Careful! Mists Can Explode,” Petroleum Refiner, Vol. 34 No. 11, 1955, pages 194–196. In Figure 1, the dew point line separates single-phase vapor from two-phase liquid and vapour regimes based on vapor pressure data. Both the lower explosive limit (LEL) and upper explosive limits (UEL) are easily measured. The existence of the air/mist flammability regime has been experimentally established and mathematically modeled for specific systems in the last half century.




