This column describes some of the aspects of chemical engineering associated with drying of organic coatingsA vs. drying of cleaning agents from part surfaces. My intent is to point out some of the problems and chemical engineering mechanisms common to both—as well as some of the differences.
DRYING OF WHAT? It matters. Water from aqueous cleaning operations or from water-based latex coatings presents very different problems and opportunities than does drying of organic solvents from vapor degreasers or from solvent-based coatings.
The major difference is due to hydrogen-bonding between adjacent water molecules. These bonds have to be broken in order for water to be vaporized.
Said another way, the heat of vaporization of water is about 8,100 BTU/gal. For n-butanol (a common solvent for organic coatings) that number is about 1,700. The ratio is ~4.8 / 1.
Those hydrogen bonds also have another effect which slows down the drying of water. They can bond to surfaces of the particles within a coating. Extra energy will be necessary to break those bonds as well.
Not that organic solvents such as n-butanol don’t form hydrogen bonds. N-butanol does, but not to the same extent as does water. In addition, water also forms polar bonds, and n-butanol only does so to about one-third the extent as does water.
In summary, drying of either water or organic solvents is going to be dominated by the need to provide energy. Drying of water will require perhaps five times more than will drying of solvents! That adds to equipment and operating cost, and slows down cycle time.
DRYING HOW?
Drying generally means evaporation of water. For drying of coatings, it very often means that.
But drying of water from parts can be done in a variety of other ways—often better ways. These don’t involve evaporation:
- Drainage, by allowing the force of gravity time to operate,
- “Knock off,” by impingement with high-velocity air, and
- Drainage, by use of centrifugal force.
None of these approaches is useful for drying of coatings.
WATCHING PAINT DRY The rate of drying of parts is limited by the rate at which heat can be transferred from hot air to the liquid, causing it to evaporate. Slow heat transfer from heated air to wet parts is normally the rate-limiting process step. Even worse, air doesn’t have a high capacity to carry heat or liquid. Consequently, huge volumes of hot air can be required.
Evaporative drying of water is also psychologically slow. Operators believe that clean parts have been produced and may be anxious to use them.
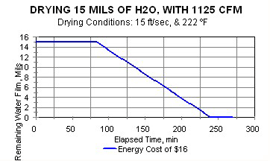
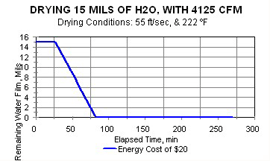
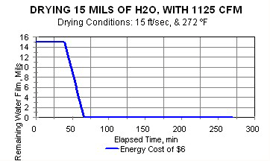
Here is a real-world example: It takes between 15 and 30 minutes to dry 100 SI of part surface wetted with 10 mils of water film, if the air is heated to around 220°F and is flowing at 1,500 SCFM. A coating line requiring that length of contact time would require construction of a new and very long building—a tunnel!
TRANSPORT PHENOMENA What occurs when cold parts or wet coatings are dried of water or organic solvent by exposure to heated air is the following:
- Large volumes of hot air are transported from a source (a heater) to be flowing alongside or impinging on the substrate surfaces.
- Heat is transferred from the rapidly moving air stream to heat the films of liquid which wet the surfaces.
- The liquid films are heated, and ultimately evaporate, when they are heated to a high enough temperature (short of the boiling point) for a long enough time.
- Heat must also be transferred from the rapidly moving air stream to heat the substrates.
The operation or step which limits the rate of removal of liquid from parts by evaporation is the transfer of heat from the hot air to the liquid films. Two factors affect that rate of removal:
- The temperature of the hot air. Higher air temperatures produce higher rates of heat transfer because the rate of heat transfer is proportional to the difference between the temperature of the hot air and the temperature of the cold (less hot) liquid films covering the parts.
- The velocity of the hot air as it moves across the part surface. Higher air velocities produce higher rates of heat transfer—chiefly by increasing the proportionality constant between temperature difference and heat transfer rate.
Both factors require a cost for energy.
Obviously, when the air is hotter more energy must be supplied to raise its temperature above the boiling point of water. Less obvious is the fact there is a cost associated with the power to drive a blower producing a higher velocity of air across part surfaces.
If there are two factors, which is more significant in producing the fastest drying rate at the cheapest cost?
PAYING FOR PARTS TO DRY There is a dominant effect of air temperature.
Hotter supply air, at the same linear air velocity, evaporates the water film much more quickly, and, therefore, the cost of drying is lowered.
This is a “double win,” as drying cycle time is shortened simultaneously with the power cost being decreased.
The effect of linear air velocity at the same air temperature is calculated and is seen by comparing Figures 1, 2, and 3.
Yes, the increase of air velocity does substantially shorten drying cycle times. But because all that extra air has to be heated, power costs increase somewhat.
The conclusion should be clear:
- Dry parts of water with heated air at the highest temperature which does not cause part damage or affect material handling after drying.
- Dry parts of water with air flow at the value which produces the required quality of dryness at the required time.
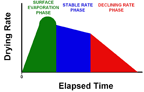
PAYING FOR PAINT TO DRY It’s only partially that way with the drying of coatings. Drying of coatings has three phases—only the first of which is the one of pure evaporation which occurs when parts are dried by vaporization.
The other two phases (which don’t occur in drying of parts) happen at a later time after the above phase—surface evaporation—has been completed.
- The second phase is where the liquid being dried passes freely through the matrix of particles that will become the solid coating when dried.
The rate-limiting step may be evaporation of liquid from the coating surface. Or it may be diffusion of the liquid through the interstices of the matrix of coating particles.
Additional applied heat always enhances drying rate during this period—as it would with drying of parts.
So, too, does additional air velocity—as it would to increase the driving force for liquid to pass through the matrix of coating particles.
- The extent to which additional heat transfer and air flow increase drying rate in this phase of drying depend upon details of the application.
The third phase of drying is where the liquid does not migrate freely through the matrix of particles that will become the solid coating when dried.
All three phases are shown in sequence in the simple block diagram as Figure 4.
In this third phase, intermolecular bonding between liquid solvent and particles can impede liquid migration, and drying rate.
Both increased air flow and air temperature can reduce cycle time in the third phase of drying. But the latter usually has the dominant effect.
DRYING VS. DRYING There are two fundamental differences between drying of parts and drying of coatings:
- First, drying of parts can be done in more than one way—evaporative, or non-evaporative. Drying of coatings is always done by evaporation of some liquid—solvent or water.
- Second, there are three different and sequential mechanisms which must be completed in drying of coatings vs. only one for drying of parts.
But where users choose to use conventional aqueous coating and cleaning technology with forced hot air drying systems, the issues are identical.
Heat transfer and air convection control evaporation of liquid from parts and of liquid from within coatings.
One will profit to understand the chemical engineering transport phenomena of heat and mass transfer upon which both applications of evaporation are based.
REFERENCES A. This column is dedicated to the memory of my good friend Ron Joseph (Dr. “Rojo”), who served as the organic coatings editor of Metal Finishing magazine for nearly 20 years, and who introduced me to the staff of the magazine. Ron helped me to prepare this column before he passed away last year.
BIO John Durkee is the author of the book Management of Industrial Cleaning Technology and Processes, published by Elsevier (ISBN 0-0804-48887). In 2012, Elsevier will publish in print his two landmark books Science and Technology of Cleaning with Solvents [ISBN 9781455731312], and Handbook of Cleaning Solvents, (ISBN-13:978145573144) as well as a 4-part e-Book Design of Solvent Cleaning Equipment. He is an independent consultant specializing in critical and metal cleaning. You can contact him at PO Box 847, Hunt, TX 78024 or 122 Ridge Road West, Hunt, TX 78024; 830-238-7610; Fax 612-677-3170; or jdurkee@precisioncleaning.com.




