The degreaser unit fails because the mixture composition in the cleaning sump is no longer the one chosen for the cleaning job. In part II of this series, I will describe how the use of certain solvent blends avoids this problem. These blends are called azeotropes.
What's an Azeotrope?
An azeotrope is a mixture of solvents that doesn't manifest the integrity problem mentioned above. But only certain combinations of solvent composition form azeotropes. Said another way, an azeotrope does not exhibit enrichment.
The reason one would want to use an azeotrope for solvent cleaning is the azeotrope would possess physical or solvency properties unavailable with a single solvent. A second reason is to avoid the aforementioned integrity problem.
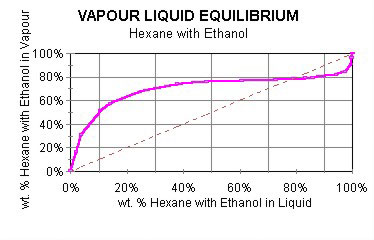
An azeotrope is a mixture of liquids that boils at a constant temperature, at a given pressure, without change of composition. In other words, an azeotrope is a multicomponent mixture that performs in distillation as if it was a single component. The reason for this observation is the liquid and vapor compositions of an azeotrope are identical.
Two or more components may be present in an azeotrope, which has a unique constant boiling point. The boiling point is not a function of composition, as noted in Figure 1 of part I. Boiling point is a function only of pressure.
The boiling point of an azeotrope may be higher or lower than the boiling points of the constituent liquids. Most azeotropes are classified as “minimum boiling,” which means the boiling point of the mixture is less than that of either component.
When the azeotropic liquid is boiled, it retains the same composition. As a consequence, the vapor has the same composition as the liquid. When an azeotropic mixture is fed to a distillation column, both the overhead (lower boiling) and bottoms (higher boiling) products have the same composition.
The constituents of an azeotrope cannot be separated by simple distillation, as is possible with most liquid mixtures. The mixture acts as if it was a single solvent, from the perspective of vaporization. In summary, an azeotrope can be used as a cleaning solvent in a conventional open-top vapor degreaser without concern that the bath composition will change when vapor is emitted from the machine.
Flexibility
There are two reasons to consider only azeotropes as cleaning solvents and ignore possible use of pure component solvents. The first reason is to avoid the situation described in part I of this column and above. The second reason is to improve cleaning quality, or add flexibility to your operations.
There are almost no soils considered single components. Recently, I learned about the composition of a proprietary coolant my client was considering. Its formulation was as complicated as an election ballot in Florida. Its developers performed a technical feat in finding a carrier fluid that could dissolve all the components.
That situation is current. The formulation of future soils will be no less complicated. We are not likely to demand a return to inferior performance of coolants, lubricants, and finishes because the performance of our cleaning processes is lagging. Azeotropes allow the cleaning solvent to have multiple capabilities (personalities), without any change in the degreasing, or solvent recovery equipment.
Azeotropes and Solubility Parameters
An azeotrope can be considered to be a single solvent, from the point of view of vaporization. Hansen solubilities for azeotropic mixtures can be calculated from the values for individual solvents and a volumetric blend rule. Azeotropic data for 90 binary mixtures of common organic cleaning solvents are given in Table I.
Each entry should be considered to be a separate solvent. This is equivalent to finding around 200 new cleaning solvents, without suppliers having to overcome regulatory and cost barriers associated with commercializing a new product. Each entry boils at a fixed temperature, which is likely different than that of either component.
Close, But No Cigar
There are some mixtures that aren’t quite azeotropes. They are called “near-azeotropes.” A near-azeotrope is a solvent mixture that boils at an almost constant temperature. There is no standard on “nearness.”
This consultant does not recommend using near-azeotropes in open-top vapor degreasers because they aren’t true azeotropes. If solvent vapor is lost, the liquid composition will change and become that of the least volatile solvent. The only question is over what time. There are plenty of azeotropes. There is no reason to consider near-azeotropes.
Use of Azeotropes
Azeotropes can contain more than two components. Ternary and quaternary azeotropes are reported in the literature. Their use only makes sense to this consultant if the third component provides some solvency for a soil component not rendered in solution by either of the components in a binary azeotrope.
The available arsenal of azeotropes greatly expands your capability to do solvent cleaning. In fact, there are far more azeotropes available than there are single solvents, which can bring value in cleaning operations. Nearly all values of Hansen Solubility Parameters (HSP) are provided for by several azeotropes.
The azeotropic solvents in Table I are “free. You can blend your own cleaning solvents by purchasing the components from any chemical supply house. Probably none of the azeotropes in Table I are available as commercial formulated products.
There are no patents on the formulations in Table I. However, you will need to add your own stabilizer package; this information will be covered in a future column.
Summary
The future of solvent cleaning is azeotropes. Reasons for this are: It is very expensive to commercialize newly identified solvents and the commercialization cost of the azeotropes noted in these two columns has essentially been paid; azeotropes can provide solvency for nearly all commercial soils; and azeotropes allow solvents with multiple HSP functionality to be used in open-top vapor degreasers whereas solvent blends should not be so used.
Notes
- The word azeotrope comes from the Greek word “zein tropos,” “constant boiling.”
- Some azeotropes do exhibit maxima in boiling points.
- While the components of an azeotrope can’t be separated by distillation, other ways can be used to separate them. One component may preferentially absorb on soil material or internal surfaces of a cleaning machine. Occasionally, one component of an azeotrope may chemically react with another material.
- Hansen Solubility Parameters (HSP) were described in this column in the January 2004 (pages 39–42) and April 2004 (pages 42–59) issues of Metal Finishing magazine.
- The volumetric blend rule is described in more detail in the forthcoming book by this author: “On Solvent Cleaning,” to be published in 2005 by Elsevier, ISBN 185617 4328.
- The data in Table I was complied from several sources: “Physical and Azeotropic Data” by G. Claxton, National Benzole and Allied Products Association (N.B.A.), 1958; www.ecosse.org/chemeng/azeotrope_bank.html; and Lange’s Handbook of Chemistry, 15th Edition, Table 5.11.
- Suppliers of refrigerants use the word “glide” to quantify how close a solvent mixture performs to being a true azeotrope. According to ANSI/ASHRAE Standard 34-1992, glide is the difference in temperature between the dew point (where the first condensate is produced) and the bubble point (where the first vapor is produced). Refrigeration is the application, not metal cleaning, where “near-azeotropes” bring the most net value.
Contact the author





