For an adhesive, sealant, or coating to be useful, it not only must withstand the mechanical forces that are acting on it, but it must also resist the service environment or the chemical forces that may also be applied. Thus, one of the most important characteristics for these materials is their endurance to corrosive environments such as those found in the chemical process industry. This article will focus on the chemical resistance of adhesives, but the fundamentals that are discussed relate to sealants, coatings, and other polymeric materials as well.
Most organic polymeric materials used to formulate adhesives, sealants, and coatings tend to be susceptible to chemicals and solvents, especially at elevated temperatures. Physical properties and durability are influenced by many environmental elements. These include high and low temperatures, moisture or relative humidity, chemical fluids, and outdoor weathering. Table 1 summarizes the relative resistance of variousadhesive types to common chemical operating environments.
In selecting polymeric materials for their chemical resistance, certain generalities must be avoided. The nature and type of chemical environments, their concentrations and temperature, the joint design, chemical resistance of the substrates, and other specific factors must be considered. As the combination of factors becomes large, formulations must become quite specialized to meet extreme requirements. As a result, understanding and planning for chemical resistance are difficult processes. Process of Degradation by Chemicals Chemical factors may affect the strength and permanence of an adhesive bond in several ways. The chemical agents may affect the cohesive (tensile strength, elongation, etc.) properties of the bulk adhesive or the adherend. Adhesive properties (shear or peel strength) at the adhesive-adherend interface could be affected as well. The extent of deterioration suffered by polymeric materials is affected by its chemical composition and structure, the composition of the acting medium, and the conditions of the action. In general, the degradation process can be physical and/or chemical.
Physical degradation occurs when the chemical environment enters the molecular network but does not react with it. For example, liquid chemicals, solvents, and moisture can diffuse into a polymer structure acting as a plasticizer. This action could cause swelling and dissolution of the polymer. More importantly, it reduces the molecular interactions within the adhesive or adherend, and, as a result, properties such as tensile strength, hardness, elongation, and heat resistance are adversely affected.
Chemical degradation occurs when the chemical environment causes a reaction that result in a breakdown of the polymer molecule. The nature of this reaction will, of course, depend on the chemistry of the polymer and the penetrating species.
The effect of moisture, especially at elevated temperature, is a good example of this. Water can cause hydrolysis of certain polymeric materials. This actually breaks the long polymer molecules down into smaller parts, and the polymer that was once a strong solid can actually be turned into a gel or liquid state.
Such processes as those described above can only occur if the chemical environment is capable of directly encountering the adhesive or adherend molecules to allow interaction. This can be divided into several steps:
- The sorption of the medium at the polymer surface
- The diffusion of the medium through the polymeric material
- The interaction of the polymer and the diffusing medium
- The diffusion and reaction products from the polymer bulk to the interface
Thus, for maximum chemical resistance it is desirable that the polymer provides a barrier to the passage of the chemical medium into its structure. Achieving a good barrier is often difficult and will depend on many parameters associated with both the adhesive, joint design, and the environment.
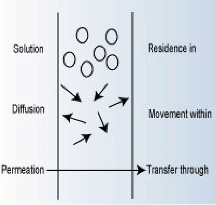
A measure of the lack of barrier resistance is generally the permeability of species through a polymer. This occurs via two processes: solution and diffusion. Figure 1 illustrates the concepts of solution, diffusion, and permeation. The chemical must first dissolve (solution) into the external surface of the polymer. It then undergoes molecular diffusion through the polymer.
However, these concepts do not cover all circumstances. Chemicals or solvents could also enter the polymer via wicking along reinforcing fibers or pigments associated with the formulation. This process is especially evident if the joint is machined or cut so that adhesive edges are exposed.
A barrier function can be incorporated into an adhesive joint in several ways:
- By choosing a low permeability polymer that is molecularly engineered to provide good barrier properties.
- By using appropriate fillers so that the penetrating species is forced to take a tortuous path into the polymer.
- By external protection. (Exposed joint edges, machined holes, etc., can be coated with a high permeability coating). In essence, there are two ways of protecting adhesives, sealants, or coatings from exposure to chemical environments: a high degree of crosslinking within the polymer structure and minimizing the amount of exposed area through design.
Test Methods Consequences of exposure to chemical environments are so severe that it is usually necessary to test preproduction joints, both in the laboratory and in the field, under conditions as close to the actual service environment as possible. The parameters that will likely affect the durability of a given joint are:
- Maximum stress level
- Average constant stress level
- Nature and type of environment
- Cyclic effects of stress and environment (rate and period)
- Time of exposure.
In applications where possible degrading elements exist, candidate adhesives must be tested under simulated service conditions. Important information, such as the maximum load that the adhesive joint will withstand for extended periods of time and the degrading effects of various chemical environments, must be addressed by several test methods.
ASTM D896, "Standard Test Method for Resistance of Adhesive Bonds to Chemical Reagents", specifies the testing of adhesive joints for resistance to solvents and chemicals. Standard chemical reagents are listed in ASTM D543, and the standard oils and fuels are given in ASTM D471. Standard test fluids and immersion conditions used by many adhesive suppliers are specified in Military Specification MMM-A-132 ( See Table 2).
Most standard tests to determine chemical resistance of adhesive joints last only 30 days or so. Unfortunately, exposure tests lasting less than 30 days are not applicable to many real-life service requirements. Practically all adhesives are resistant to these fluids over short time periods and at room temperatures. Some epoxy adhesives even show an increase in strength during aging in fuel or oil over short time periods. This effect is possibly due either to post-curing or slight plasticizing of the epoxy by the oil.
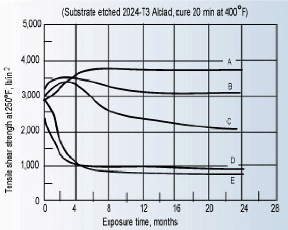
Figure 2 shows the long-term effect of a heat-cured epoxy joint to various chemical environments. As can be seen, the temperature of the immersion medium is a significant factor in the aging properties of the adhesive. As the temperature increases, more fluid is absorbed by the adhesive, and the degradation rate increases.
The effect of simultaneous exposure to both mechanical stress and a chemical environment is often more severe than the sum of each factor taken separately. Mechanical stress, elevated temperatures, and exposure to chemicals or solvents can be a fatal combination for certain adhesives and sealants if all occur at the same time. The stress can act to accelerate the degradation caused by the environment, and vice versa. Joints that will be exposed to both chemical environments and load at the same time are especially vulnerable and prototype specimens need to be appropriately tested. Epoxy Adhesives When compared to other structural adhesives, those based on epoxy chemistry have outstanding resistance to solvents, weak acids, and alkali compounds. This accounts for their use as adhesives, sealants, and coatings in many industrial applications. As a result, epoxy compounds are used to assemble water softener tanks, chemical containers, brewery tanks, and other industrial components in the chemical industry.
Epoxy adhesives are generally more resistant to a wide variety of liquid environments than other structural adhesives. However, the resistance to a specific environment is greatly dependent on the type of epoxy curing agent used and the formulation employed. Generally, those factors tending to promote thermal stability also tend to improve chemical resistance. So very often the adhesives having the greatest thermal stability will also provide the greatest chemical resistance.
| Adhesives | Hot Water | Acid | Alkali | Oil, Grease | Fuels | Alcohols | Ketones | Esters | Aromatics |
| Cyanoacrylate | 6 | 6 | 6 | 3 | 3 | 5 | 5 | 5 | 4 |
| Epoxy + polyamine | 2 | 2 | 2 | 2 | 3 | 1 | 6 | 6 | 1 |
| Epoxy + anhydride | 3 | 2 | 2 | 2 | 2 | 6 | 6 | 2 | |
| Epoxy + polyamide | 6 | 3 | 6 | 2 | 2 | 1 | 6 | 6 | 3 |
| Acrylic | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 3 |
| Acrylate acid diester | 4 | 6 | 6 | 3 | 3 | 5 | 5 | 5 | 4 |
| Polyurethane | 3 | 3 | 3 | 2 | 2 | 2 | 5 | 6 | - |
| Silicone (RTV) | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 |
| Epoxy - polysulfide | 6 | 2 | 2 | 2 | 2 | 2 | 6 | 6 | 2 |
| Epoxy - phenolic | 2 | 2 | 2 | 3 | 3 | 2 | 6 | 6 | 2 |
| Conditions, 77°F after: |
| 30 days in RT water 30 days in 110? humidity cabinet (100% RH) 30 days in 95°F salt-spray cabinet (5% salt) 7 days in JP-4 jet fuel 7 days in anti-icing fluid (isopropyl alcohol) 7 days in hydraulic oil (MIL-H-5606) 7 days in hydrocarbon test fluid (70/30 v/v isooctane/toluene) |
References:
- Weggemans, D. M., "Adhesives Charts", in Adhesion and Adhesives, Vol. 2, Elsevier, Amsterdam, 1967.
- Aerospace Adhesive EA 929, Hysol Division, Dexter Corp. Tech. Bull.
BIO Edward M. Petrie is the sole proprietor of EMP Solutions, a Cary, N.C.-based consulting firm focused on solving problems in the adhesives and sealants industry. He also works as a technical expert for SpecialChem. For more information, visit www.specialchem4adhesives.com.




