

Industrial and consumer demand growth have tapped nearly all sources of fresh water available and driven the cost of water steadily higher.1,2 Industries that depend on water have been forced to become more efficient, or they will price themselves out of business.
In addition, facilities that find they must discharge pollutants are stuck between a rock and a hard place. Should they concentrate wastes and risk exceeding concentration limits or use the increasingly expensive “solution to pollution is dilution” method? The metal finishing industry has historically been a heavy user of water resources, so plants have been working hard on methods to avoid these poor choices by making processes more flexible and efficient.
Significant strides have been made the last 20 years in limiting the water consumption of the various finishing processes and cutting discharge of environmental pollutants by implementing several changes in process operation. These actions, in the context of increasingly scarce fresh water supplies and tough environmental regulations, have been both cost effective and socially responsible. Future reductions in waste and water usage, however, are likely to require extensive use of simple analytical devices such as electrical conductivity meters.
These meters, which automatically adapt to changing conditions, verify the cleanliness of water for reuse and required rinse flows needed to clean metal workpieces. Conductivity meters have been shown to reduce water consumption by up to 40% without additional labor cost or extensive process modification.3
Metal Surface Treatment
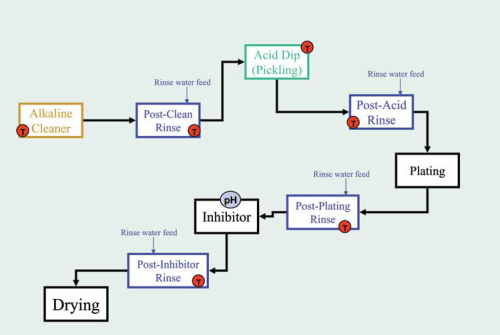
Metal finishing can consist of many different physical and chemical steps used to produce metal surfaces of specific texture. Typical processes include pickling, electroplating, anodizing, coating, and mechanical surface preparation. A representative process flow diagram is shown in Figure 1.
The initial step is cleaning either in a tank or a spray washer. The cleaning solution is usually slightly alkaline and frequently a proprietary recipe of various surfactants. The next step will rinse the cleaner and any dragout soils from the previous stage. Further steps depend on the type of processing desired.
In electroplating, the metal surface will be treated with a strong acid (pickling) solution to remove the impure surface layer, then rinsed, soaked in the plating solution, rinsed again, soaked in a rust inhibitor, rinsed yet again, and then dried. Throughout the process stages, workpieces carry off (dragout) small amounts of unwanted oils, greases, and chemicals as they leave these processes. Dragout can quickly cause problems downstream by neutralizing downstream baths, passivating the work surface, producing undesired spotting, or etching the workpiece. It is quite common to rinse the workpiece immediately prior to each treatment stage.
Efficient rinsing attempts to remove about 99.9% of contamination with the minimum required outlay of water and energy. This can also be thought of as diluting the remaining contaminant by a factor of 1,000. Rinsewater is the largest category of water consumption for metal finishers and has the largest potential for use reduction and water reclaim.
Improving Rinsing Requirements
The first step in improving rinsing requirements is minimizing dragout from process baths. Process bath design modification can be very helpful here. Steps to minimize dragout include:
- Lowering bath concentrations;
- Increasing bath temperature to lower the solution’s viscosity;
- Using wetting agents to reduce the solution’s surface tension;
- Removing workpieces slowly and allowing them to drain on racks;
- Using spray rinses or air knives above the process baths;
- Installing drainage boards after process baths to return dragout to the baths.
Typically, some bath chemicals will still adhere to the workpiece. Indeed, some care may be needed to prevent excessive contaminants from building up in the process baths since they are typically removed in the dragout process. Regular monitoring of the process baths may be necessary.
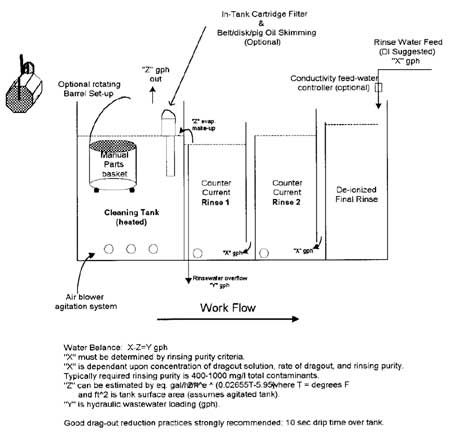
Rinse tank design is optimized when all the water is used to remove as much of the contaminants as possible. The rinse tank itself should prevent short-circuiting of makeup water since clean water that leaves the tank without mixing is wasted. A spray rinse is an excellent way to pretreat the workpiece before soaking in the tank. Multiple tanks are useful for improving the removal efficiency since the second tank will have a much lower contaminant level.
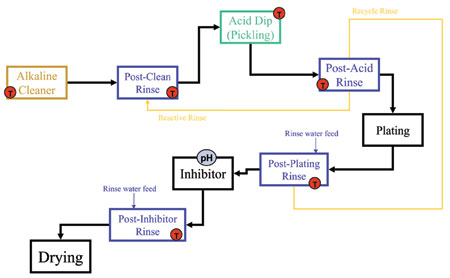
Fresh water is traditionally used for rinsing purposes, but examination of the entire process can reveal water streams that can be reused to improve efficiency. Countercurrent rinsing (Figure 2), where the freshest water does not contact the work piece until the last rinse stage, is a technique that can effectively use the same rinsewater twice, though it is a little more complex to install. Reusing an acid rinse as makeup water for an alkaline rinse (reactive rinsing) has also been shown (Figure 3) to improve efficiency by reducing the need for makeup water.
Other methods for water use reduction in rinse tanks include flow restrictors to prevent excessive rinsing; flow cut-off valves for when the process is not active; and flow meters to monitor excessive water consumption. The Metal Finishing Guidebook has extensive information on implementing these and other design modifications for rinse tanks.4
Tank design and static operational changes are appropriate for stable processes, but more advanced techniques are necessary during startup of a new product, or when processes are likely to change conditions. An excellent way to control rinsewater flows and purity under dynamic conditions is to monitor the solution’s electrical conductivity.
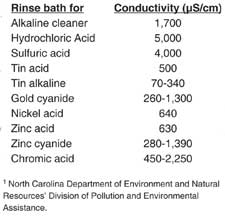
Conductivity is a nonspecific measurement of the ionic contamination in the rinsewater. Although acids and bases will have higher conductivity, the salt residue on metal workpieces will also contribute to the conductivity. As the salt accumulates in the rinsewater, the solution will have higher conductivity. Conductivity meters read out in units of microsiemens/cm (µS/cm) or micromhos/cm (µohms/cm), which can usually be correlated with a total dissolved solids (TDS) concentration in ppm for any given application.
Conductivity meters minimize the use of makeup water by monitoring the rinsewater bath and only allowing fresh water when the conductivity reaches a certain set point. The set point depends on the chemical being rinsed and the desired cleanliness of the workpiece (Figure 4). For example, fresh water may have a conductivity of 100 to 300 µS/cm while initial rinsewater of 1,000 to 2,000 µS/cm may be considered adequate. For a typical cost of $1,000 to $2,000 per measurement point, the meter can reduce water consumption by 40% and have an economic payback of about one year.3
Conductivity Sensors
There are three major types of conductivity sensors: two-electrode, four-electrode, and toroidal. The simplest design (Figure 5) involves two metal electrodes that are separated by a plastic insulator. These sensors are accurate but designed for measurement over specific, relatively narrow ranges of conductivity (contaminant level).
The cell constant of the sensor determines the range of operation, with higher values reserved for measuring more concentrated solutions.
Cell constants of 0.01 to 1.0 are common and correspond to measuring conductivity from less than 1 µS/cm to more than 2,000 µS/cm. Two-electrode sensors are ideal for measuring clean water solutions, such as in reverse osmosis (RO) or deionizer applications, but subject to measurement interference when the contaminant level is higher or when there are substances that can coat or foul the electrodes. The signal from an electrode conductivity sensor is proportional to the surface area of the electrode, so coating can be a severe measurement problem. Nonetheless, RO water is being recommended more and more for improved makeup water, so two-electrode sensors are frequently found on rinse tanks.
Four-electrode sensors use a pair of counter-electrodes to compensate for the coating and polarizing effects present at higher contaminant levels. They are much more rangeable than their two-electrode cousins, with only one cell constant (typically near 1.0) necessary for most applications. They are mainly used for concentrated solutions of acids, bases, or salts that require measuring higher conductivity levels in solutions that do not have large amounts of suspended solids.
Toroidal (a.k.a. inductive or electrodeless) conductivity sensors do not have any exposed metal parts and consist of two wire-wrapped rings (toroids) encased in plastic (Figure 6). An electrical signal is passed through the transmitting toroid and produces current flow in the surrounding solution, which is measured by the other (receiver) toroid and converted to a conductivity reading based on the signal received.
Toroidal sensors are much more resistant to coating and ideal for applications in strong plating solutions and spent rinsewaters.
Conductivity users should be aware that temperature has a substantial effect on the conductivity reading. Higher temperature increases the mobility of all ions in solutions and, therefore, the conductivity also. The conductivity sensor should measure temperature and the meter should compensate the reading to a reference temperature (25°C is standard) with an adjustable setting. A simple 2%/°C adjustment is sufficient for rinse baths, though measurements of concentrated solutions may benefit from a more customized setting.
Correlating the conductivity reading to a ppm (mg/l) concentration in a rinse bath can be a little challenging since each type of ion in the solution has a different characteristic conductivity. In natural water, the most common ions are sodium and chloride, so total dissolved solids (TDS) are assumed to be salt (sodium chloride). Rinsewaters, however, represent dilute versions of the process baths, and may have a different relationship between conductivity and ppm. These differences can usually be worked out by using adjustment factors that allow the meter to display customizable concentration values on the display.
Conductivity measurement with toroidal sensors is nearly maintenance free and can provide many years of service if the equipment is properly installed. The sensor should be totally submersed in the liquid and kept at least one inch away from tank walls. Gas bubbles will cause the reading to drop, so locate the sensor away from any air agitation.
In addition to monitoring rinsewater, conductivity meters can be useful in controlling the concentration in process baths. Many process baths involve contacting the work piece with an ionic solution of a known concentration.
A good example would be the “acid dip” or pickling stage. The acid dip removes surface metal from the piece, using up the acid and putting metal salts in solution. The acid concentration must, however, be maintained to keep the process operating efficiently.
The need to add makeup acid into the tank can be monitored with conductivity despite the fact the metal salts and acid contribute to the conductivity reading. Since the acid has much higher conductivity than the salt, a net drop in conductivity can be used as the trigger for makeup acid. Online control of the makeup acid can be construed as another way to save on rinsing costs since the acid tank can run at a lower concentration, involving less dragout and a reduced requirement to rinse.
Conclusions
Metal finishing processes require a high level of water consumption, but in this age of water conservation for both economic and social responsibility reasons, it’s important that metal finishers find ways to reduce water usage. By using conductivity analysis effectively, plants can obtain a significant reduction in water usage and costs.
References
- www.waterindustry.org
- The Price of Water Trends in OECD Countries, Organisation for Economic Co-operation and Development, ISBN 9264173994; 1999
- North Carolina Department of Environmental and Natural Resources Division of Pollution and Environmental Assistance.
- Metal Finishing Guidebook & Directory, Vol. 102, No. 4A, Elsevier Inc., New York; 2004.
Contact the author




