Researchers at the US Department of Energy's Ames Laboratory and Iowa State University have developed a 3D printing process that can create a chemically active catalytic object in a single step. This opens the door to more efficient ways to produce catalysts for complex chemical reactions in a wide range of industries. The researchers describe the process in a paper in ACS Catalysis.
While 3D printing has found applications in many areas, its use for controlling chemical reactions, or catalysis, is relatively new. Current production of 3D catalysts typically involves various methods for depositing the chemically active agents onto pre-printed structures.
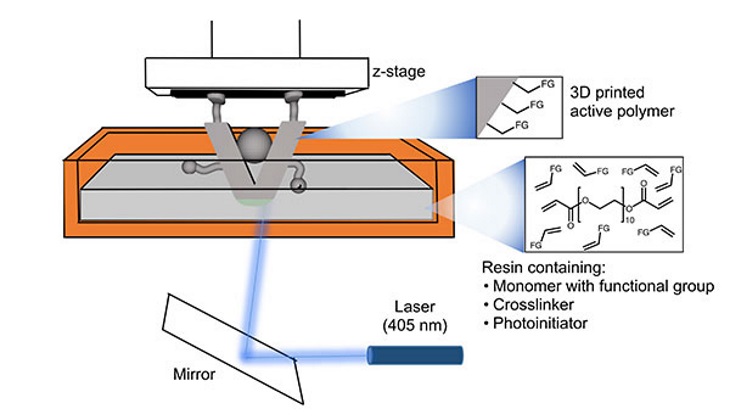
The Ames Laboratory method combines structure and chemistry in a single step using inexpensive commercial 3D printers. The structures are designed in a computer and built directly by shining a laser through a bath of customized resins made up of monomers that assemble the 3D structures and catalytic active sites such as carboxylic acid, amine and copper carboxylate groups. The laser causes the resins to polymerize and harden layer-by-layer, producing a final product with intrinsic catalytic properties.
"The monomers, or building blocks that we start with, are designed to be bifunctional. They react with light to harden into the three-dimensional structure, and still retain active sites for chemical reactions to occur," explained Sebastián Manzano, a graduate student in the Department of Chemistry at Iowa State University, who conducted most of the experiments.
The catalysts built with this method demonstrated success in several reactions common to organic chemistry. They are also adaptable with further post-processing, making multi-step reactions possible.
"We can control the shape of the structure itself, what we call the macroscale features; and the design of the catalyst, the nanoscale features, at the same time", said Igor Slowing, a scientist in heterogeneous catalysis at Ames Laboratory. "This opens up many possibilities to rapidly produce structures custom designed to perform a variety of chemical conversions."
This story is adapted from material from Ames Laboratory, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.


