The new material, called a “nanoscoop” because its shape resembles a cone with a scoop of ice cream on top, can withstand the extremely high rates of charge and discharge that can the cause conventional electrodes used in Li-ion batteries to rapidly deteriorate and fail.
The nanomaterial could thus lead to the development of high-power, high-capacity Li-ion rechargeable batteries. It could also be used in electric automobiles, as well as batteries for laptop computers, mobile phones, and other portable devices.
The team of researchers, led by Professor Nikhil Koratkar at Rensselaer Polytechnic Institute, New York, USA, charged and discharged a nanoscoop electrode at a rate 40 to 60 times faster than conventional battery anodes, while maintaining a comparable energy density.
“By using our nanoscoops as the anode architecture for Li-ion rechargeable batteries, [charging a laptop or cell phone in a few minutes] is a very real prospect,” said Koratkar. “Moreover, this technology could potentially be ramped up to suit the demanding needs of batteries for electric automobiles.”
The anode structure of a Li-ion battery physically grows and shrinks as the battery charges or discharges. When charging, the addition of Li ions increases the volume of the anode, while discharging has the opposite effect. These volume changes result in a buildup of stress in the anode. Too great a stress that builds up too quickly, as in the case of a battery charging or discharging at high speeds, can cause the battery to fail prematurely. This is why most batteries in today’s portable electronic devices like cell phones and laptops charge very slowly – the slow charge rate is intentional and designed to protect the battery from stress-induced damage.
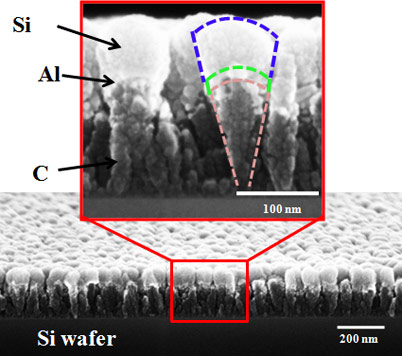
The Rensselaer team’s nanoscoop, however, was engineered to withstand this buildup of stress. Made from a carbon (C) nanorod base topped with a thin layer of nanoscale aluminum (Al) and a “scoop” of nanoscale silicon (Si), the structures are flexible and able to quickly accept and discharge Li ions at extremely fast rates without sustaining significant damage. The segmented structure of the nanoscoop allows the strain to be gradually transferred from the C base to the Al layer, and finally to the Si scoop. This natural strain gradation provides for a less abrupt transition in stress across the material interfaces, leading to improved structural integrity of the electrode.
The nanoscale size of the scoop is also vital since nanostructures are less prone to cracking than bulk materials, according to Koratkar.
“Due to their nanoscale size, our nanoscoops can soak and release Li at high rates far more effectively than the macroscale anodes used in today’s Li-ion batteries,” he said.
A limitation of the nanoscoop architecture is the relatively low total mass of the electrode, Koratkar said. To solve this, the team’s next steps are to try growing longer scoops with greater mass, or develop a method for stacking layers of nanoscoops on top of each other. Another possibility the team is exploring includes growing the nanoscoops on large flexible substrates that can be rolled or shaped to fit along the contours or chassis of the automobile.
This study was supported by the National Science Foundation (NSF) and the New York State Energy Research and Development Authority (NYSERDA).




