Previous attempts to make the batteries using other methods have led to reduced power, according to the researchers.
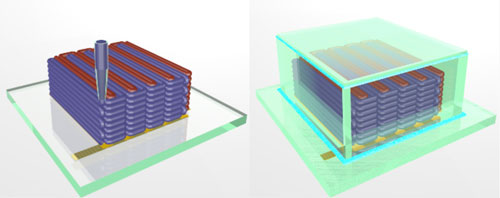
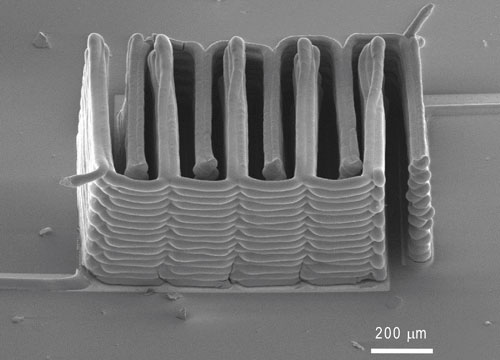
To make the microbatteries, a team based at Harvard University and the University of Illinois at Urbana-Champaign printed precisely interlaced stacks of tiny battery electrodes, each less than the width of a human hair.
To print 3D electrodes, the group first created and tested several specialized inks. Unlike the ink in an office inkjet printer, which comes out as droplets of liquid that wet the page, the inks developed for extrusion-based 3D printing must fulfill two difficult requirements. They must exit fine nozzles like toothpaste from a tube, and they must immediately harden into their final form.
In this case, the inks also had to function as electrochemically active materials to create working anodes and cathodes, and they had to harden into layers that are as narrow as those produced by thin-film manufacturing methods. To accomplish these goals, the researchers created an ink for the anode with nanoparticles of one lithium metal oxide compound, and an ink for the cathode from nanoparticles of another. The printer deposited the inks onto the teeth of two gold combs, creating a tightly interlaced stack of anodes and cathodes. Then the researchers packaged the electrodes into a tiny container and filled it with an electrolyte solution to complete the battery.
They measured how much energy could be packed into the tiny batteries, how much power they could deliver, and how long they held a charge. “The electrochemical performance is comparable to commercial batteries in terms of charge and discharge rate, cycle life and energy densities," said Shen Dillon, assistant professor of materials science and engineering at University of Illinois at Urbana-Champaign, who co-authored the project. "We’re just able to achieve this on a much smaller scale,”
The printed microbatteries could supply electricity to tiny devices in fields from medicine to communications.
“Not only did we demonstrate for the first time that we can 3D-print a battery; we demonstrated it in the most rigorous way,” said Jennifer A Lewis, senior author of the study, who is also the Hansjörg Wyss professor of biologically inspired engineering at the Harvard School of Engineering and Applied Sciences (SEAS), and a core faculty member of the Wyss Institute for Biologically Inspired Engineering at Harvard University.
The results have been published online in the journal Advanced Materials.
Find us on Google? Click here to sign up to receive our weekly e-newsletter and get news sent directly to your inbox!




