Cumulative research over the last 30 years has since proven a broad range of VCI products on the market today to be perfectly safe. This same body of research has, unfortunately, also proven that certain specific VCI formulations can, in fact, be toxic.
Responsible manufacturers have, of course, been removing potentially hazardous products from the market as they become suspect. A considerable amount of confusion and misinformation, however, remains among members of the metalworking community, since the published research regarding the health and safety of VCI technology is difficult to understand for anyone who is not an expert in this area of chemistry. Therefore, the remainder of this article will summarize the historical development of VCI technology and clarify the body of health and safety research on a more basic level, in order to dispel existing myths as well as promote the continued application of safe VCI products in the future.
Early VCI Technology
Globalization has meant that most component manufacturers ship metal parts to assembly plants around the world. The success of these shipments, in turn, hinges upon protecting parts from the destructive effects of rust caused by extreme climatic stresses common to long ocean voyages and storage in staging warehouses. Traditionally, coating metal parts with waxy oils provided this rust protection. Cost reduction and environmental concerns, however, have led most manufacturers to abandon this antiquated method in favor of the current standard that involves shipping their products “dry,” without oil, in VCI plastic packaging.
This type of packaging generally consists of polyethylene film impregnated with chemical formulations unique to each manufacturer. While the underlying formulations can vary significantly, the finished products all function similarly in that they release very low concentrations (typically in parts per ten-thousandth) of invisible corrosion inhibiting vapors into the surrounding air. The vapor molecules subsequently condense onto exposed metal surfaces and form a molecular corrosion shield that can protect against rust and other forms of corrosion for five years, and even longer in some cases.
When the VCI packaging is later removed, all vapor corrosion-inhibiting molecules rapidly evaporate. This leaves the metal parts clean and ready for immediate use.
Like most innovations, VCI technology has undergone several generations of product evolution before reaching its current level. The first generation appeared in 1937, when Shell Oil Co. introduced a product with the trademark name of “Dichan” to the market. Dichan, or more appropriately, dicyclohexylamine-nitrite (also known as dicyclohexylammonium-nitrite), was described in the patent as a “vapor phase corrosion inhibitor” that had sufficient vapor pressure to release corrosion-inhibiting molecules into its surrounding atmosphere.
Other companies licensed dicyclohexylamine-nitrite from Shell and began to coat it on paper, either by itself or as a delivery system for a range of other corrosion inhibitors. These licensors shortened the technical name to VPI, VPCI, and also “vapor corrosion inhibitor” or “VCI,” and launched VCI paper as well as other VCI coatings and powders.
A market was developed for VCI products with applications requiring limited corrosion protection for up to a few months. This application range and market size were limited, however, by inherent weaknesses of the product. Due to its high vapor pressure, dicyclohexylamine-nitrite diffused away rather quickly and tended to flake off when coated onto paper. Furthermore, the hydrophilic (water absorbing) properties of VCI paper tended to hold corrosion-accelerating moisture in direct contact with the very metal surfaces the product was supposed to protect. A further blow that limited expansion of this initial phase of VCI technology was the revelation in the early 1970s that Shell dicyclohexylamine-nitrite, the foundation of most then-existing VCI formulations, was a carcinogen.
Risks of Secondary Amines
Due to their many useful properties, amines as a whole have been used for decades as the principal components of a full range of metalworking products, including some VCI formulations. This is despite the fact that most amines were known to be mildly toxic as well as skin, eye, and respiratory irritants. Historically, these safety concerns were controlled by ensuring workers use gloves and safety glasses in properly ventilated workshops.
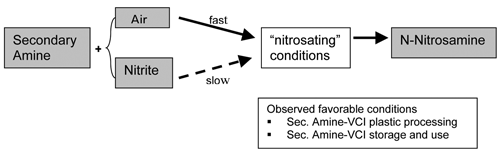
However, this proved to be no longer sufficient in the early 1970s, when researchers began to suspect that secondary amines, a subset of the amine family, were carcinogenic. These early researchers correctly determined that secondary amines readily convert into carcinogenic N-nitrosamines when exposed to “nitrosating” agents (Figure 1). When initial findings regarding the health and safety of secondary amines first surfaced, responsible manufacturers stopped making products that combined secondary amines and nitrosating agents, including “nitrites,” in their formulations. The first new hazardous material to be targeted this way was dicyclohexylamine-nitrite, the direct combination of a secondary amine with a nitrosating agent, used in so many VCI formulations at the time.
Subsequent advances in research in the early 1990s showed that using secondary amines without nitrites was still not safe. Researchers came to understand the carcinogenic N-nitrosoamines are most likely to form when secondary amines combined with nitrogen oxides. Nitrogen oxides are a fundamental component of the air we breathe and actually more concentrated in the air of cities and industrial environments. In short, they’re everywhere amines might be used. Even worse, by 2001, German researchers confirmed that some N-nitrosoamines, including those generated by dicyclohexylamine-nitrite, were not only carcinogenic, but genotoxic as well.1,2
Increased health and safety concerns led a fair number of VCI product manufacturers to abandon secondary amines altogether in favor of primary and tertiary amines. This approach subsequently proved to be flawed, however, since technical (commercial) grades of primary and tertiary amines are generally not pure, containing 10% or more secondary amines. Once heat is applied during manufacturing processes, these components of relatively “safe” amines may also form into N-nitrosamines.
The conversion of secondary amines may also continue during the storage and application of the resulting products.3 Evidence of these amine conversions was found in a German study, in which 40 commercially available VCI products were chemically analyzed.4 It was found that 23 out of 40, or slightly more than half of the products purchased on the market at the time, tested positive for the presence of secondary amines, and some of these products contained carcinogenic N-nitrosamines. The other 17 products were nitrite-based and free of amines and nitrosamine byproducts. These products were pronounced completely safe, based on all current scientific findings and worldwide regulations.
All of this accumulated scientific evidence is being incorporated into evolving health and safety regulations worldwide, the most recent being German Technical Regulation TRGS-615, which went into effect in late 2003. TRGS-615 is specifically written to protect factory workers from exposure to carcinogenic and possibly genotoxic N-nitrosamines. It seeks to accomplish this by prohibiting all metalworking products, including VCI, that contain secondary amines or hidden secondary amines. This prohibition included known useful corrosion inhibitors like di-amine carboxylates, di-amine benzoates, diethanolamines, and dicyclohexylamine.
Today, more than 30 years after the initial discovery that combinations of secondary amines and nitrosating agents can form hazardous N-nitrosoamines, a false association still persists that “nitrites” in VCI products are dangerous. The research summarized above, however, has clearly proven the reverse to be true. It is the secondary amines in all metalworking and VCI products that pose the risk. At the same time, there are still many safe amine-based products on the market.
Nitrites Usage in VCI Products
In the late 1970s, VCI product manufacturers were at a peak in their search for safe and effective new components they could incorporate into their formulations. Dicyclohexylamine-nitrite, the foundation of so many VCI formulations in the past, had recently been identified as a carcinogen (later as a genotoxin) and abandoned by responsible VCI product manufacturers. Many manufacturers tried developing alternative amine-based formulations without nitrites. Still others, wisely, decided to take their search in the opposite direction and took a closer look at what nitrites had to offer.
In 1979, Northern Technologies International Corp. (NTIC) made two significant innovations. NTIC not only managed to introduce a new VCI chemical system to the market that was founded on common food additives, but was also the first to successfully impregnate these VCI formulations into plastic films on a commercial basis.5 Today, subsequent generations of these initial innovations are still being successfully marketed by NTIC in almost 50 countries under the Zerust or Excor brands. Of particular significance is Zerust and Excor products do not contain any amines. Rather, they rely on nitrites, specifically sodium nitrite, as the foundation of their VCI rust inhibitor systems.
The value of sodium nitrite as a contact corrosion inhibitor has long been known. Nevertheless, conservative chemists had not considered its utility in a VCI system, due to an extremely low vapor pressure that was almost immeasurable at that time. The efficacy of sodium nitrite in VCI formulations has, however, since been proven in thousands of commercial and military applications worldwide, as well as recognized standard test methods for vapor corrosion inhibitors used by U.S. and NATO military organizations.6
The unique low-vapor pressure of sodium nitrite also contributes to much longer-lasting corrosion protection compared to higher vapor pressure amine-based alternatives. In addition to its proven efficacy in rust protection, the use of sodium nitrite in VCI products also has an excellent health and safety record on three separate tiers.
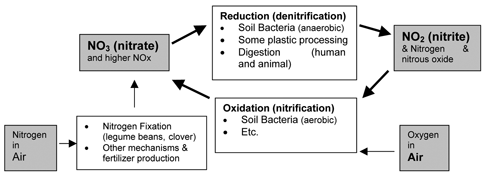
First of all, the safety of sodium nitrite as a chemical substance is particularly well studied and monitored because of its primary use as a popular and critical preservative for meats.7 There is evidently no alternative that can prevent the growth of the bacterium that causes botulism poisoning. Nitrites are also an integral part of the human and animal digestion of foods, particularly vegetables. Saliva and stomach acids convert nitrates from food into nitrites as part of the overall “nitrogen cycle,” the most important nutrient cycle in ecosystems (Figure 2). Recent research findings in Sweden and England confirm the discovery of antimicrobial effects of nitrites and this nitrate-nitrite cycle on bacteria responsible for stomach infections and tooth decay. Current research also seeks therapies based on these findings about nitrite chemistry. Microbiologist Ferric Fang of the University of Washington is reported to have said, “we’ve gone from considering all of these things to be toxic and carcinogenic to realizing that [nitrites are] playing a fundamental hemeostatic role.”8
Nitrites, as with most chemicals, can be poisonous if the pure material is ingested in sufficient quantities. However, quantities distributed in Zerust and Excor packaging films, for example, approach the low levels found in foods. Furthermore, rather than being used as a loose powder, nitrites, and other VCI additives are imbedded (melted) into the plastic, effectively locking in all but what appears on the surface.4,9
Consequently, they are well below safe permitted levels for humans and animals, whether by eating a VCI packaging product directly, or by breathing trace amounts of VCI molecules released into the air. That is why safety regulations, such as the German TRGS-615, restate the long-standing acceptability of corrosion inhibitors that contain less than 1% of their weight in nitrites.
Secondly, unlike amines, the low levels of nitrites in Zerust and Excor VCI packaging, for example, do not cause eye, skin, or respiratory irritations. Therefore, these VCI products do not require additional safety gear, such as gloves, respirators, or safety glasses, and will not cause any harm despite prolonged direct contact with skin, eyes, or mucous membranes. The use of gloves is still highly recommended when handling metal parts, however, to protect them from corrosive acids emanating from human skin.
Finally, there was considerable concern the “nitrites” in these VCI films would readily combine with secondary amine residues found on metal parts and subsequently form carcinogenic N-nitrosoamines. Several studies tried to deliberately create this effect in a laboratory setting. However, they failed in each case, proving the use of nitrite-based VCI films is also safe under these conditions.
Health and Safety of VCI Products
As stated earlier, certain VCI formulations and products have been proven hazardous via the accumulation of a rigorous body of scientific evidence. That, of course, is no reason to falsely condemn VCI technology as a whole.
All VCI manufacturers and products are subjected to continuous, rigorous screening to ensure worker safety is at the highest level.
Remember, the ranks of VCI product manufacturers include several respected “blue-chip” public companies. Regardless of size, however, all chemical companies are subject to the laws and regulations that have been established worldwide and continually updated as on-going research provides new insights into protection of individual health and the environment. Secondly, to preempt frivolous lawsuits that are so pervasive these days, corporations go to extreme lengths to ensure product safety by subjecting any new formulations to rigorous toxicological testing and legal review before releasing them for sale.
Once offered for sale, these same products are screened yet again for safety by governmental and certifying agencies in various countries. Finally, as a third level of rigor and scrutiny, industrial chemical products are reviewed by in-house toxicology departments of all major manufacturing companies and military services before they are approved for use by employees of these organizations. At all three levels cited here, a complete disclosure of all raw materials in a product formulation is required. Consequently, a VCI manufacturer could not even begin to sell any product without first having successfully navigated all of these hurdles.
In the meantime, interested readers can consult the references at the end of this article, which show a few scientific papers and reviews about specific VCI additives and formulations that have been tested, as well as reviews by agencies such as the U.S. Environmental Protection Agency (EPA), California EPA, National Toxicology Center, and their counterparts in other countries, particularly in the European Union, Japan, and Russia.
Conclusions
Significant advances have been made in the performance and scientific understanding of VCIs. These advances also support evolving governmental regulations that protect worker health and safety. In particular, it is clear the use of secondary amines is hazardous, both alone (in the presence of air) and in molecular combination with other “nitrosating agents.” It is also clear the class of corrosion inhibitors that uses nitrites without the presence of amines is safe and has proven for several decades to give effective long-term corrosion protection.
VCI product manufacturers include “blue chip” companies as well as other responsible public and private firms that are not only subject to existing laws and regulations, but also actively respond to advances in scientific knowledge. Such companies ensure product safety by subjecting new products to rigorous toxicological testing and legal review. Once offered for sale, there is ongoing monitoring of product safety as well as associated safety labeling and documentation by governmental and certifying agencies in the various countries.
Additional rigorous reviews are performed by in-house toxicology departments of all major manufacturing companies and military services, including detailed reviews of formulations and sometimes even confirmatory chemical analyses of actual products. These hurdles and reviews, as well as the intent to provide products and services with integrity, are a realistic basis for understanding the current acceptability of VCIs and related corrosion inhibitors.
References
- Gebel, T.W.; Müller, M.M.; G.A.; Hallier, E., Archives Toxicol., 75; 604–608; 2000
- Shanina, Y.L., Reviews on Corrosion Inhibitor Science and Technology, NACE Press; March 2004
- Reinhard, G.; Lautner, S.; Hallierc, E., Zbl. F. Arbeitsmedizin Bd. (Heidelberg), 50(12):404–410; 2000
- Rocker, M.; Boveleth, W.; Spigelhalder, I.; Breuer, D., Gefahrstoffe - Reinhaltung der Luft (Springer VDI Verlag), 63(5):187–191; 2003
- Boerwinkle, F.P.; Kubik, D.A., U.S. Patent 4,290,912; 1981
- Laboratory tests of basic VCI functions are called vapor inhibiting ability (“VIA”) or “jar” tests, including U.S. Federal Standard 101C, Method 4031 (also referred to U.S. Military specifications), German Standard TL 8135-0002 (also referred to in German and NATO military specifications) and Japanese Industrial Standard JIS Z 1519. Laboratory humidity chamber simulations of accelerated climatic conditions related to shipping and long-term storage of packaged metal parts are done in conditions of temperature and humidity described in ASTM D1735 and IEC 60068-2-30
- Institute of Food Technologists Annual Meeting, paper session, “Recent Advances in the Safety Assessment of Sodium Nitrite and Cured Meats,” New Orleans; June 2001; D.L. Archer, Paper 6033, Session 64-1, “Microbiological Importance of Sodium Nitrite in Assuring Cured Meat Safety”; J.R. Coughlin, Paper 6031, Session 64-2, “Reproductive and Development Effects of Sodium Nitrite: The California Proposition 65 Challenge; E.E. McConnell, Paper 6032, Session 64-3, “Animal Cancer Studies of Sodium Nitrite: Historical Review and Recent National Toxicology Program Bioassay Results”; J.M. Riley, Paper 6034, Session 64-4, “Sodium Nitrite’s Safety and Efficacy: Communication Challenges”; S.A. Miller, L.M. Crawford, Paper 6035, Session 64-5, “Sodium Nitrite as a Food Additive: Safety and Regulatory Considerations; http://ift.confex.com/ift/2001/techprogram/session_751.htm
- Scientific American, 291(3):24; September 2004
- Westphal, G.A.; Müller, M.M.; Herting, C.; Bünger, J.; Hallier, E., Archives Toxicol. 75:118–122; 2000
E-mail the authors
The November 2004 issue of Metal Finishing published two papers on the topic of vapor corrosion inhibitors (VCIs). Randy Dutton has been concerned for quite some time that consumers do not adequately understand the potential health and safety issues regarding VCIs, and he asked if Metal Finishing could bring this to the attention of our readers. We decided to also look for a second article that would provide another overview of this topic. James Henderson agreed to write an overview of the different chemistries that are used for purposes of corrosion inhibition. Henderson is aware of the controversy that exists in his industry and explains how the different acid gas scavenging films work and which of them pose heath threats, while others apparently do not. These two articles do not reflect the opinion of Metal Finishing or Elsevier Inc. To those of our readers who purchase or use VCIs, we urge you to read both papers with open eyes. If you have any residual concerns, we request that you investigate further, or contact one or both authors for more information. |





