
Early adopters have been using these advanced pretreatments for several years now, and chemical suppliers are on their second and even third generation of products. The automotive industry is using these pretreatments on bodies, and the U.S. Military is taking a very close look as well. There are a number of phosphate-replacement advanced pretreatments available today. Most are based, at least in part, on zirconium. (Zirconium has been used as a replacement for chromium coatings on aluminum for many years, but it’s only in the past several years that its use on steel has become more common.) The chief issues with earlier-generation zirconium-only pretreatments has been overcoming flash rust and poor paint performance. One proven method to avoid these shortcomings is the addition of organic ingredients, such as silanes or phosphonates.By now, most finishers are aware of the important advantages of switching to these advanced pretreatments, but let me first review them, then tell you what these new pretreatments are, and, finally, cover what you need to know before you take the leap.
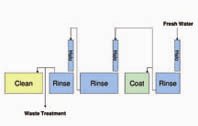
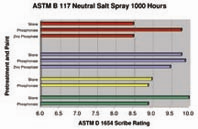
Why Make the Leap? Advantages. The new advanced pretreatments are environmentally friendly because they: have no zinc and have either no phosphorous or low phosphorous; provide energy savings because they operate at ambient temperature; require less capital investment since fewer stages are needed; and cost less to charge the bath because a smaller tank can be used. They are more simple to operate because they have wide operating windows, produce almost no sludge and no scale, and have simple waste treatment. They are versatile since they work with most substrates and paints, and they match the paint performance of the phosphate process they replace. The bottom line is cost savings. Let’s look at some of these advantages a little more closely.Today, phosphorous is coming under growing discharge restrictions from regulatory authorities. Why? Because phosphorous discharge to surface waters causes eutrophication, the overgrowth of phosphorous-nourished plant life. This starves the water of oxygen, which eventually kills fish and other animal life. Left unchecked, eutrophication can lead to the complete filling in of a body of water by plant life.The Clean Water Act of 1972 regulates waste water discharges to surface waters. However, it doesn’t restrict phosphorous discharge. Instead, phosphorous discharge limits have been put in place by local authorities in a myriad of regions across North America, especially in environmentally sensitive watershed areas such as the Chesapeake Bay area in Virginia, the Spokane River area in Washington, the Great Lakes and Hudson River areas of New York, and the Chattahoochee River area in Georgia. Often, the publicly owned treatment works facilities in these regions will restrict phosphorous discharge through surcharges. How much phosphorous is too much? Many of these regions have limits or surcharges that range from as high as 25 mg/l to as low as 1 mg/l of phosphorous. To give a point of reference, a conventional iron phosphate bath might contain 500–1,000 mg/l of phosphorous and the rinse stage after it may contain15–30 mg/l, assuming a 3% contamination level in the rinse.Besides phosphorous discharge restrictions, there are other issues with conventional pretreatments. Zinc phosphates contain heavy metals, such as zinc and nickel—the discharge of which is regulated by the Clean Water Act. These metals also pose hazards in the workplace. For example, certain forms of nickel are now classified as human carcinogens. Advanced pretreatments operate at ambient temperature, which saves on the cost of heating. However, conventional phosphate pretreatments operate at elevated temperatures, and with energy prices as volatile as they are today, it’s a risk that most metal finishers would be happy to avoid. Using calculations that spray washer manufacturers employ when designing new equipment, a reduction in temperature from 130°F to ambient temperature in a typical washer spraying 400 gallons per minute of solution from a 1,200-gallon tank running two shifts per day, with a natural gas cost of $6 per million BTU would save almost $25,000 annually in natural gas.Fewer chemical stages are required with advanced pretreatments, since a final seal is not used. The process sequence for advanced pretreatments starts with a thorough removal of organic soils, such as mill oils and metalworking fluids as well as inorganic contaminants such as smut, rust, mill scale, laser scale, weld scale, etc. Next is to thoroughly rinse, preferably double counterflowing rinsing with good quality water in order to prevent contamination of the pretreatment bath. Next, the pretreatment is applied to the clean substrate, and in the last stage it is rinsed with good quality water. (Often, reverse osmosis, or RO water, is recommended if the tap water is not of good quality). A good sequence, therefore, would be: clean–rinse–rinse–pretreatment–rinse. This is the recommended sequence for those building a new pretreatment line. It is possible, through the use of fresh water exit risers, to use advanced pretreatments in a conventional, five-stage spray washer or immersion line. For this conversion, it makes sense to put the advanced pretreatment in the fourth stage (See Figure 1). Many five-stage lines have a fourth stage that is about 30–45 seconds long. This contact time is adequate for advanced pretreatments. This also saves on cost since a smaller tank is used for the initial charge, compared to a typical (larger) third stage. With the proper use of exit halos and cascading, savings of 50% in water usage has been demonstrated.These advanced pretreatments can be applied by either spray or immersion in a line constructed of stainless steel alloy 304L or 316L or polypropylene. Mild steel is less desirable, not only because these pretreatments operate at a pH of approximately 4–5, but also because the pretreatments are reactive with mild steel, and exposure to a mild steel tank over a long shutdown could reduce the bath life stability. Phosphonate advanced pretreatments, however, can be successfully operated in mild steel spray washers, provided the bath chemistry is closely controlled. Compared to iron, and more so zinc phosphate, these advanced pretreatments have wide, robust operating windows. For example, a typical zinc phosphate has a total acid window of 25–30 ml. This is an operating window of 7% either side of the midpoint. By comparison, the concentration operating window of advanced pretreatments is a huge 25–33% either side of the midpoint.Say goodbye to sludge and scale reaction by-products. Why? Conventional iron and zinc phosphate pretreatments operate at a high acid content (total acid titration) and elevated temperature, leading to significant dissolution of the substrate. Iron dissolved into the phosphate bath is oxidized from the ferrous state to the ferric state where—because of its low solubility—it precipitates as ferric phosphate sludge. This is the heavy sludge commonly seen inside the pretreatment system. Because ferric phosphate has lower solubility at high temperature, it also forms scale on heating surfaces. This can lead to maintenance headaches and a loss of heating efficiency. Advanced pretreatments, on the other hand, operate at about one-tenth the total acid value of a zinc phosphate, or about one-half the total acid of an iron phosphate. They also operate at ambient temperature, which may be as much as 60°F (32°C) cooler than a phosphate. The low total acid and ambient temperature operation of advanced pretreatments result in almost no sludge formation (See Figure 2). Because they contain no inorganic phosphate, advanced pretreatments do not form scale on heaters.Because these advanced pretreatments are free of regulated heavy metals, free or nearly free of phosphorous, and are biodegradable, waste treatment is simple and inexpensive. For rinse water, most finishers can simply adjust the pH to limits set by local authorities and discharge. If in-house treatment is needed, treatment with lime to a pH of 8–10 will precipitate virtually all of the ingredients. Rinse water can even be recycled after precipitation with lime, filtration and ion exchange, leading to a closed-loop pretreatment.How do advanced pretreatments perform under organic coatings? They’ve been extensively tested by numerous major manufacturers. In particular, silane pretreatments have been tested by major U.S. and European automotive manufacturers and proven to meet virtually all of their requirements. In another example, under various paint systems, both silane and phosphonate pretreatments on steel substrates have proven to match the performance of a zinc phosphate. In Figure 3, the first set of bars in red is a cathodic epoxy electrocoat primer with a TGIC powder topcoat; the second set of bars in blue is a TGIC powder coat finish; the third set of bars in yellow is a cathodic epoxy electrocoat primer with a chemical agent resistant coating (CARC) topcoat; and the fourth set of bars in green is a powder epoxy primer with a CARC topcoat. Of course, not every combination of substrate and paint will provide the same performance, so it’s important to test your particular system.
Some Basic Advanced Pretreatment ChemistryLet’s look at silanes first. A simple silane molecule consists of a silicon atom combined with an organic molecule. For paint pretreatment, however, more complex silanes described as “organofunctional” are used. Through proper selection of the organic constituents used in the silane molecule, the chemist creates an organofunctional silane molecule, which reacts and forms stable bonds with both metal hydroxides on the substrate and organic groups on paint resins. When these organofunctional silanes are reacted with water during the pretreatment supplier’s manufacturing process, they form what are called “polycondensates.” These retain the paint and metal-bonding properties of the silane, but in an easy-to-use form. The polycondensate is the safe chemical form in which “silane” products are usually made commercially available to metal finishers. In use, as the silane film dries on the pretreated substrate, neighboring hydroxyl groups on the silane molecule react with each other to form a dense cross-linked network. Finally, in order to further enhance performance, non-regulated group IV-B metals, such as zirconium, are used to selectively and preferentially bond to the metal substrate, providing improved corrosion-resistance compared to a silane-only process. The composition of these group IV-B metals in the silane product is carefully balanced in order to provide the optimized deposition rate of the metal onto the substrate, which maximizes paint performance. In effect, a dual coating is formed in one step: an inorganic coating comprised of zirconium and other unregulated metals, and an organofunctional silane coating. During coating dry off and/or paint cure, the silane coating crosslinks to provide a durable robust coating.Phosphonate coatings are made of select organic phosphonate molecules along with titanium, zirconium and proprietary activating ingredients. The phosphonate molecule forms a protective, corrosion-inhibiting film on the metallic substrate, maintains its stability in hard water and has limited complexation with hard water salts. The phosphonate, titanium and zirconium concentrations are carefully balanced in order to optimize paint performance. Somewhat like the silane process, the phosphonate process forms a combination organic and inorganic coating in a single stage.Silanes and phosphonates work well with all common metallic substrates, such as steel, aluminum, zinc, magnesium and their alloys. They also perform well with all conventional powder coats, such as TGIC polyester, TGIC-free polyester, epoxy, hybrids and others, as well as with liquid and electrocoat paints. They’ve also been proven to perform extremely well in rubber-to-metal bonding.
Making the LeapWhen you’re ready to leap off the fence to an advanced pretreatment, it’s important to start with some careful planning. This requires good communication with your equipment, pretreatment and paint suppliers. End-item performance requirements as well as limitations—such as cost, substrates, water quality, paint, equipment materials of construction, and number of stages—should be communicated clearly to all.Next, decide which advanced pretreatment you will use. In order to decide, you need to consider washer materials of construction, water quality, phosphorous discharge limits, and paint performance.The performance test used to select the advanced pretreatment should be applicable to the end use. Salt spray is not always the best test to use. For end items that will be used in a dry indoor application, cross hatch adhesion may be all that’s necessary. For zinc substrates, certain cyclic corrosion tests may be more applicable. However, neutral salt spray may be a good test to employ for items that will be used in a marine environment.For corrosion testing, it is important to follow these guidelines in testing: Test both the current and the advanced pretreatment processes side by side on the same substrate, and include lab control panels. Coat all panels with the same paint, test all panels in the same test cabinet and test all panels for one time period. This ensures that no extraneous variables are introduced to the test, which could lead to confusing results. Finally, use replicate panels for better confidence in the results. Next, look at your existing pretreatment line to see if modifications are necessary. Make sure you have good water quality. Calcium, chloride, sulfate, and other hard water salts can react with or reduce performance of pretreatments. Purchasing a reverse osmosis system is usually a good investment for cost savings and improved quality. In many cases, however, especially with phosphonate pretreatments, good quality tap water—with a conductivity of less than about 250 µS/cm—has been used successfully.Excellent cleaning and rinsing are needed with advanced pretreatments. Soils and contaminants from the cleaner, such as phosphorous, silicate and complexants, can cause problems if dragged into the pretreatment. Therefore, a good washer “tune-up” should be conducted before starting up an advanced pretreatment. Plan and implement any needed washer modifications, such as installing or rerouting exit halos and/or counterflows. Order all special titration supplies and automation equipment early. Chemically descale all stages and rinse thoroughly. Consider replacing nozzles if they are worn, then carefully align them. Once the switch is made, operator training is needed. The coatings may have a different appearance. The advanced pretreatment will have different testing methods, controls and bath adjustments. Depending on the product used, there may be a titration that is specific to zirconium, or there may be photometric methods for titanium and phosphonate. These methods give accurate measurement of the active ingredients, rather than using a simple acid-base titration which, although simple, can be influenced by water quality and cleaner drag-in.It’s also a good idea to post SPC charts of pertinent operating parameters near the pretreatment line. This gives everyone involved with the finishing process a good visual history of how the line is running and of any trends developing.
The Bottom LineAdvanced pretreatment replacements for conventional iron and zinc phosphates have proven to be robust performers. They are environmentally friendly, save energy and simplify pretreatment, maintenance and waste treatment operations. They are effective on most metallic substrates and with most paints. Finally, they save overall operating costs.
BIO Gary Nelson is Product Manager, Surface Treatment, at Chemetall, New Providence, NJ. He has a Bachelors in Chemical Engineering and a Masters in Business Administration. He has more than 25 years experience in the metal finishing industry. He can be reached at gary.nelson@chemetall.com.




