
Although industrial dust explosions are usually linked to processes involving organic compounds, handling metal powders can produce similar conditions involving static electricity. Contact charging, or tribocharging, and the build-up of charges on product as well as equipment and installations are factors in the potentially lethal mix. The list of possibly pyrophoric metal powders includes iron, aluminium, platinum, titanium, magnesium, cobalt and nickel. And as the industry ponders the industrial-scale application of nanotechnology and the enhanced activity associated with ever-smaller particles, the list of potentially pyrophoric candidates gets longer.
While the current focus of interest in the United States is the sugar industry, a series of incidents in the past five years indicated that guidelines issued by the National Fire Prevention Association were being widely flouted. That prompted a US Chemical Safety and Hazard Investigation Board report which recommended the adoption of a combustible dust standard for general industry. The effort is directed at all industries where this hazard may exist and is being supported by Congressional action.
Bulk materials are handled industrially in large quantities and at high transfer rates. Filling and emptying operations are often carried out using flexible intermediate bulk containers or other vessels made of insulating plastics. These factors enhance the possible build-up of a high enough level of charge accumulation to cause an incendiary discharge.
Many metals powders that are potentially reactive are covered by a thin layer of oxide which passivates them. They only become hazardous if the film is disrupted chemically, by milling, or by abrasion during transfer.
If a static charge grows enough to initiate a “discharge by ionisation” of the surrounding atmosphere, the hot plasma within the discharge channel may ignite the flammable dust cloud. The coincidence of a sensitive dust cloud in air with a discharge of sufficient energy has led to many serious fires and explosions in different industries. German figures suggest that dust explosions are common in German industry, and that 10% of those blasts are caused by static electricity.
Dust explosions can occur if flammable solid material is divided into fine particles dispersed in air. Fine particles may also be formed during handling and processing of granules due to abrasion. The finer the particles, the more sensitive the dust cloud with regard to ignition sources and the more violent the explosion. For a worst case assessment, the fraction below a mesh size of 63μm is usually chosen. At atmospheric conditions, dust explosions normally show the following characteristics:
• Explosion range: from 20g/m3 to several kg/m3;
• Maximum explosion overpressure: 9 bar for organic material and up to 13 bar for metal powder;
• Maximum rate of pressure rise in a one cubic metre vessel: 100–300 bar/sec for organic material and up to approximately 1000 bar/sec for metal powder.
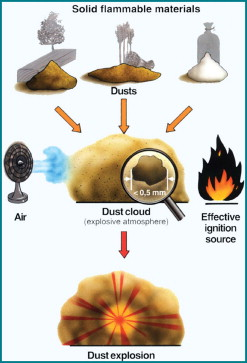
The combination factors needed for a dust explosion are shown diagrammatically in Figure 1. Figure 2 shows the quite spectacular effects of an unconfined dust explosion using about 6kg of maize starch homogenously dispersed in air. With regards to human safety, dust explosions are normally even more hazardous than a gas explosion because exposure to heat radiation lasts much longer.
A fire or explosion may take place if coincidence occurs between a flammable material, oxygen and an ignition source. These factors are the three components of the well-known “fire triangle”. To prevent an explosion, one of these components must be excluded. The most important objective for industry is to avoid an explosive atmosphere at all.
However, despite the need to avoid explosive atmospheres, most industrial operations involving powders and granules take place under atmospheric conditions. The exclusion of effective ignition sources such as static electricity then represents the only basis for safety.
Assessment of the occurrence and incendiary potential of discharges in different situations represents in practice the most important, but also the most difficult step in the analysis of electrostatic hazards. Because of the difficulty, a more or less phenomenological approach is commonly used. Discharges occurring in practice are classified into different types. These have different incendiary potentials. The electrical properties of the products and installations, their geometric arrangement and the operation determine which discharge type will occur in a given situation.
Apart from spark discharges, other types include brush discharges of various kinds, cone and corona discharges, all of which are capable of initiating a dust explosion under the right conditions.




