
Surface treatments are key technologies used in the fight against corrosion for many industrial applications. Surface coatings allow the bulk properties of one metal to be combined with the surface properties of another. Coatings extend in-service component life through improved corrosion protection, enhanced aesthetic appeal, and greater wear resistance.Low-cost, bulk-applied, zinc-based coatings applied to steel substrates are widely specified for anti-corrosion, and benefit from the many unique properties of compounds containing hexavalent chromium (Figure 1).
For electroplated zinc and zinc alloys, this takes the form of a post-plated film applied by immersion from an aqueous chromate of acids and hexavalent chromium compounds. In addition to extending time to oxide formation (white rust), a range of distinctive colors, such as clear-blue, yellow, green, and black, are obtained.
Chromate was first patented in 19361 and largely commercialized during the 1950s.2 The basic technology has remained essentially unchanged, but it is well documented that hexavalent chromium compounds are environmentally and toxicologically hazardous.
Environmental Change
Alternatives to hexavalent conversion coatings have existed since the late 1970s. These were based on the less- toxic trivalent chromium compounds and limited primarily to low-performing, blue-bright-type coatings. Over the course of the last several years, there has been growing interest in hexavalent chromuim-free processes. This is due, in part, to the new ELV and WEEE legislation, which dictates that vehicles and electronic components sold in Europe can no longer contain hexavalent chromium.
The End of Life Vehicle (ELV) Directive is the name given to the European Union legislation 2000/53/EC of September 18, 2000, concerned with preventing waste generated by vehicles below 3.5 tons' total permissible weight, through the re-use, recycling, and recovery of end-of-life vehicles and their components (Figure 2).
The directive has been the subject of much discussion and numerous articles over the past few years, but essentially it sets out for member states to encourage vehicle manufacturers to limit the use of hazardous substances.
After July 1, 2003, member states are to ensure that materials used do not contain cadmium, lead, mercury, and hexavalent chromium. Listed in Annex II are exemptions from this directive for these materials used in certain defined applications. In corrosion-preventative coatings, Annex II listed that a maximum level of hexavalent chromium at 2 g per vehicle would be allowed. This, therefore, will impact the hexavalent chromium materials found in surface coatings, such as chromate films, on zinc electroplating. Not affected by the directive implications are hexavalent chromium materials used in chemical processing (which are regulated through other workplace and environmental hazard related directives). Therefore, chromium electroplate is not restricted in the ELV directive.
Annex II of the directive was amended June 27, 2002; the hexavalent chromium replacement date was changed to July 1, 2007, with the following notes:
- A maximum concentration value up to 0.1% by weight and per homogeneous material, for...hexavalent chromium…shall be tolerated, provided these substances are not intentionally introduced;
- and "intentionally introduced" shall mean "deliberately utilized in the formulation of a material or component…"
A further amendment to Annex II was published on September 20, 2005. It now makes the provision under exemption item 13 as follows:
- Corrosion preventive coatings—July 1, 2007; and
- corrosion preventive coatings related to bolt and nut assemblies for chassis applications—July 1, 2008.
At press time, OEM response to this change was unclear. But several sources suggest that existing hexavalent chromium-free implementation plans will proceed to meet the 2007 target date.
Trivalent Blue
Many are familiar with the clear, blue color films on electroplated zinc, which have been successfully obtained from formulations based upon inorganic trivalent chromium compounds for a number of years. They were originally developed to overcome discoloration caused by the reaction of hexavalent chromates with metallic contamination (notably iron) from non-cyanide-containing zinc electrolytes and patented in 1978.3 These were thin films around 30 to 40 nm thick as confirmed by SEM and XPS techniques, and offered a moderate level of corrosion resistance for zinc. Further research directed towards the use of metal ions, notably cobalt, were reported, providing improvements to these early formulations, patented in 1982.4
These chemistries have developed further in reliability and performance in recent times, having found particular favor with alkaline non-cyanide zinc users. Their increased material cost and process control requirements are balanced by their longevity, color consistency, and higher corrosion resistance. Today, improved decorative aspects for blue passivate films are available from trivalent chromium formulations that are free from fluoride and cobalt. They are very tolerant to iron levels in the passivate solution, producing lower thickness films with enhanced blue bright color and moderate corrosion protection. Improvements with corrosion resistance for blue passivates can be obtained from formulations containing a higher chromium concentration combined with fluoride, cobalt, and other corrosion inhibitors.
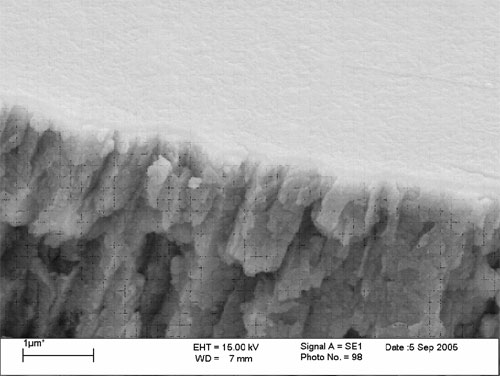
These build increased film thickness onto zinc around 75 to 100 nm without micro cracking as confirmed by FE SEM (Figures 3 and 4). Neutral salt spray demonstrates improvements in their value as corrosion-resistant films.
Trivalent Iridescent
As end user performance requirements increased for trivalent passivates, notably from the automotive OEMs, recent times have seen a focus for the adoption of "thick film" iridescent. Chemistry developments were reported and patented that used higher trivalent chromium contents with cobalt and organic acids.5,6 Combined with operation at elevated solution temperatures of 55 to 65°C, these conditions built significant thickness onto zinc. The thicker film of around 300 nm is responsible for the recognizable red-green iridescent color; this type of iridescent passivate compares favorably with that achieved from yellow chromates.
Many reports have confirmed their improvements against heating as automotive OEMs demand deposit performance with and without heating at typical levels such as 120°C/1h, 120°C/24h or 150°C/2h, dependent upon the individual manufacturer concerned. It has long been established that chromates do not perform well against temperature, as their cracked films tend to dehydrate and lose most of their protective value. The thicker iridescent trivalent films do not suffer from this and, therefore, offer enhanced protection.
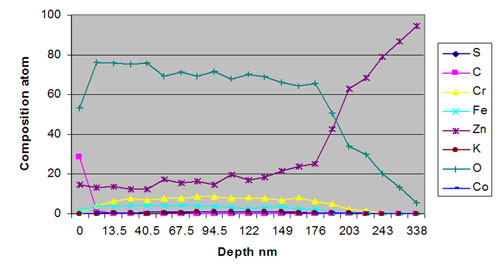
Surface characteristics of the thick-film passivate has been confirmed by SEM and XPS, and have shown the significant role played by zinc contained within the passivate matrix. Neutral salt spray evaluations have demonstrated that comparable or improved corrosion resistance is achieved from the thick-film iridescent passivate.
Low-Temperature Iridescent
The most recent development is from a new type of trivalent chromium-based formulation being commercially introduced. It provides the characteristic iridescent color but from more acceptable processing conditions. The significant change is higher solution operating temperatures are no longer required to build film thickness onto zinc, thereby saving valuable energy consumption and contributing to a reduction in overall process costs.
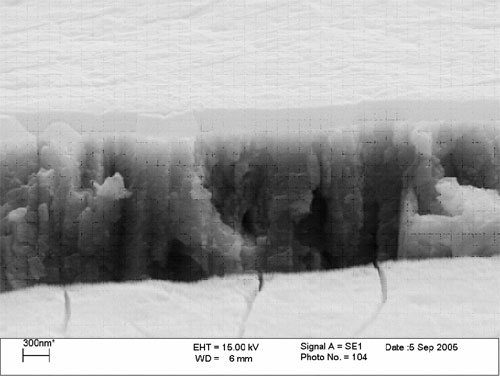
Surface analysis has shown film thickness around 250 nm with no micro cracking to the surface (Figure 5). Corrosion studies demonstrate competitive performance against existing trivalent passivates and that yellow chromates can be directly replaced.
Trivalent Black
Highly decorative black chromates from pigments, typically silver-containing chemistries, have been traditionally used on zinc. The incorporation of silver has the effect of decreasing corrosion protection to moderate levels while increasing material and processing costs. The development of zinc alloys in the late 1980s allowed the use of non-silver chromates to achieve functional black finishes at lower cost.
Developments in the mid 1990s showed that zinc alloy electrodeposits of zinc iron and zinc nickel were readily blackened from phosphate-based trivalent chromium passivate, and were patented in the U.S. in 1995.7,8 It has emerged that, to meet current market expectations, these are now only competitive when used with a topcoat, to achieve appearance and corrosion resistance.
Market-suitable black passivates for use on zinc have proven more elusive. Early attempts using molybdates could provide a functional color and appearance, but without achieving appreciable corrosion resistance. Research has continued and some commercial products are now being adopted that offer appearance and performance onto zinc when used with a suitable topcoat.
Topcoats
The reliability of trivalent passivate films is improved by the use of post-applied topcoats based upon silicated or organic chemistries (Figure 6). These modify and further enhance the functional properties of the passivate films such as appearance, abrasion resistance, and corrosion protection.
Inorganic seals form a glassy layer with passivate and have been adopted for lower corrosion demands when cost is the significant driver. They have been chosen for applications where fluid compatibility is an issue, such as automotive braking systems. Decorative demands are met with the use of organic-based formulations that apply highly glossy, clear films in excess of 500-nm thickness.
For fastener applications, custom torque 'n' tension topcoats are available that modify surface tribology to ensure uniform torque and clamping characteristics, which are essential for joint integrity in modern automated assembly.
Applicator Considerations
There are several important challenges associated with the introduction of processes that are free of hexavalent chromium; the change for applicators is significant. Hexavalent chromium technologies have proven to be extremely flexible and tolerant, demanding only a low level of control and equipment. In the vast majority of applications, hexavalent chromates have received only minimal process control measures. Proprietary products for the formation of chromates have been reliable and inexpensive; it has been proven cost effective to replace them on a regular basis, negating the need for prolonged solution life.
The transition from hexavalent to trivalent technology has been significant, not only from an environmental standpoint, but process application as well. Operating parameters that were previously ignored now must be carefully controlled and monitored. Additionally, more rigorous performance requirements have been added to specifications, placing an even greater burden on both platers and chemical suppliers.
As a result, a partnership has been formed where the plater and chemical supplier work together to achieve the lowest operating cost while ensuring maximum performance from the final coating. This also directly benefits the end user by assuring a consistent quality output from a specific applicator.
An example of how chemical suppliers and applicators work as partners can be found in an innovative concept known as ZinKlad. The ZinKlad program was developed by MacDermid and launched into the market in 1999 as a vehicle to effectively manage the transition away from hexavalent chrome. This unique program combines the use of innovative chemistry with process control and strict audit protocols, resulting in a global network of approved applicators.
The latest zinc-based application technology is matched to synergistic hexavalent chromium-free passivates and topcoats to produce a hexavalent chrome-free, high-performance coating.
Initial Audit
A complete audit of the applicator's facility, production line, process, and quality controls is an essential first step in effective change management. The process sequence is reviewed to assess suitability for inclusion of trivalent passivate and/or topcoat. In addition, process and quality control procedures must be in place to ensure proper bath chemistry is maintained and parts are routinely tested against minimum performance requirements.
Line decontamination is necessary for the provision of hexavalent chromium-free finishing. This would take the form of thorough water rinsing of process equipment such as tanks and racks/barrels, followed by chemical treatment.
In the case of an existing process line conversion, care should be taken with alkaline pretreatment stages, as they will probably contain chromate residues. Therefore, it is prudent to discard and make up with fresh chemistry.
The absence of hexavalent chromium can be checked and, once verified, the line is signed-off and ready for installation of the new chemistry.
Passivate Control
To achieve maximum cost-effective performance from the trivalent chromium technologies, a defined program of control procedures has proven beneficial. The applicator makes a provision to monitor and control the passivate solution temperature, solution pH, and immersion time. Solution concentration is determined through various methods of analysis, the results typically being calculated in ml/L. Adjustments to the concentration would typically replace drag-out losses, as only minor amounts are actually consumed during film formation.
It has become beneficial to monitor zinc and iron levels as they build as contaminants in the passivate solution during operation. Dissolved metallics have a significant effect on final deposit appearance and corrosive protective value. Elevated levels of ferrous and ferric iron significantly influenced the film appearance and reduced corrosion-resistant properties.
Once the necessary process modifications have been made, each approved applicator is required to submit samples from production for independent performance monitoring. This is done both for initial applicator approval and on a quarterly basis thereafter. In order to ensure maximum consistency, all samples from the ZinKlad network are channeled through to a central validation point.
Every batch of samples is logged and then assessed against the same evaluation criteria, specifically appearance, thickness, freedom from hexavalent chromium, corrosion resistance, and, when specified, torque tension.
Freedom from hexavalent chromium is important to verify the applicator has deposits free from the presence of detectable hexavalent chromium and also to demonstrate ongoing process reliability. This is laboratory checked against ISO 3613 and supports the regular on-site testing at the applicator’s facility. This method defined leaching for five minutes in boiling deionized water and uses a dipenylcarbazide reaction, which is oxidized in the presence of hexavalent chromium to form a red complex, which can be quantified by US/VIS spectrometry. Corrosion resistance is evaluated by neutral salt spray to ASTM B-117. A range of performance finishes are specified with defined minimum attainment levels both with and without heating treating at 120°C for four hours.
The torque 'n' tension characteristics for standard threaded fasteners can be evaluated, as defined by the end-user finish specification. Unless otherwise specified, samples are checked against the default procedure of SAE/USCAR-11.
The ZinKlad approval program has continued to find acceptance with a large number of global OEMs, and ZinKlad finishes have been approved against a number of specifications.
The first global OEM to benefit from a managed applicator program was the Ford Motor Co. Their engineering material standard WSS-M21P17-B3 for worldwide fastener coating suffix S437 exclusively specifies ZinKlad 250.
Conclusions
Environmental and legislative pressures have been the catalyst for adopting alternative technologies, to enable the substitution of hazardous substances, such as hexavalent chromium, that have been used in traditional processing. Trivalent chromium-based formulations have proven to be competitive and are already adopted into the supply chain. Topcoats that enhance and modify the functional properties of the deposits are sometimes needed to meet increasing end-user demands.
Experience to date with this significant technology change has illustrated the need to have a strategic co-operation between all companies involved in the supply chain. The use of approved applicators enables the introduction of quality assurance protocols to manage the initial process change, monitor in-process controls, and verify on-going deposit performance. This ensures that quality levels are maintained, providing a high level of end-user confidence. MacDermid has demonstrated that a network of approved applicators has been extremely useful in assisting end users to adopt a truly global strategy to meet supply chain demands.
A greater level of mutual understanding and support means that industry will be better equipped to handle changing social, economic, and environmental issues in the future.
References
- Wilhelm, E.J., U.S. Patent 203580; 1936.
- Johnson, D., U.S. Patent 2559878; 1951.
- Bishop, Foley, Frank, U.S. Patent 0417123; 1978.
- Crotty 1982, U.S. Patent 4359348; 1982.
- Klos, U.S. Patent 5368655; 1994.
- Preikschart, Jansen, Hulser, WO97/40208.
- Bishop, C.V., U.S. Patent 5,393,354; 1994.
- Bishop, C.V., et. al., U.S. Patent 5,415,702.
Bio
Michael Wyrostek is North American manager for MacDermid's Global Functional Coating Group.





