

AbstractSILVERONTM GT-101 Bright Silver, an improved cyanide-free silver electroplating bath, has been recently commercialized. A series of successful industrial installations have demonstrated that the silver deposits obtained from the SILVERON GT-101 bath are suitable for a very wide variety of applications. The present article describes the characteristics of the deposits from this cyanide-free, alkaline silver electrolyte in comparison to those obtained from conventional cyanide silver electrolytes. Properties—including the deposit appearance, grain structure, adhesion to copper and nickel substrates, corrosion and tarnish resistance, electrical contact resistance, ductility, solderability, wire bonding capability, surface reflectance, micro hardness and friction/wear behavior—are discussed in detail.IntroductionElectroplated silver, as a plated finish, has been very widely used by many different industries, owing to its attractive appearance, excellent electrical and thermal conductivity, high resistance to oxidation and compatibility with consumer applications requiring contact with skin, as well as its relatively low cost compared to gold or palladium. Up to this point, cyanide-based silver electrolytes are predominantly used, although a few cyanide-free silver plating processes have become available in recent years. A number of reported drawbacks of the existing cyanide-free silver electrolytes have limited their acceptance as an alternative to conventional cyanide-based plating processes, including unsatisfactory deposit appearance, electrolyte instability, difficulties in bath maintenance, limited plating rate and relatively high cost. There can be no doubt that cyanide is a unique Ag(I) ligand, and to develop a cyanide-free silver electrolyte capable of providing comparable performance to that of a cyanide-based silver electrolyte is extremely challenging. Intensive development work on new cyanide-free silver electrolytes at Dow EM during the last years has been focused on fundamental studies of silver electrodeposition with a variety of different silver complexing agents in comparison to cyanide, followed by selection of the best complexing agent in conjunction with the use of compatible bath additives based on both the electrolyte performance and extensive deposit characterization. The SILVERONTM GT-101 electrolyte, an improved cyanide-free silver plating system, was finally tested under production conditions, in barrel, rack and reel-to-reel plating processes. The industrial tests demonstrated that the new cyanide-free silver electrolyte is very chemically stable and provides consistent plating performance. The resulting silver deposit is suitable for a very broad range of applications. In this article the characteristics of the new cyanide-free silver electrolyte, and the performance of the silver deposit it produces are discussed in detail. Some limitations of the process are also reviewed. Characteristics of the SILVERON™ GT-101 Silver ElectrolyteThis alkaline silver electrolyte comprises silver ions, a cyanide-free complexing agent for Ag(I), organic grain refiners, pH buffering agents and bath additives to extend plating capability at higher current densities. The operating parameters of SILVERONTM GT-101 Bright Silver are shown in Table 1. As mentioned earlier, much of the development effort was focused on choosing the optimum complexing agents for Ag(I) from a large number of candidates, based on both the electrolyte performance and deposit characteristics. The complexing agents selected for use in GT-101 electrolyte allowed excellent solution stability to be attained. The maximum plating rate of the electrolyte, as shown in Table 1, is 10 A/dm2 in reel-to-reel plating applications and up to 15 A/dm2 in jet or wire plating. Note that, in spite of all efforts made in optimizing complexing agents, bath additives and conductivity ions, the maximum usable current density is still significantly lower than that a cyanide-based electrolyte can achieve. Substantially increased polarization during silver deposition was seen in electrochemical studies when using any of the cyanide-free complexing agents tested, versus cyanide. As a result, when plating at the same current density, the deposition potential for a cyanide-free electrolyte is substantially higher compared to that of cyanide electrolytes. This leads to a much more pronounced negative influence of the hydrogen evolution when using cyanide-free complexing agents, resulted in lower capability for high speed plating. Hence, a compromise in plating rate needs to be made, when selecting a cyanide-free silver plating process, or when converting a cyanide-based process to a cyanide-free process.
One of very few known technical drawbacks of cyanide-based silver electrolytes is the accumulation of carbonate, an electrolytic decomposition product of cyanide during production. In order to extend bath life, carbonate needs to be removed from cyanide-based silver electrolytes. Unlike cyanide, the complexing agents used in GT-101 electrolyte are chemically stable and no decomposition product formation was observed either during development or during more one year of production test.
Generally, soluble silver anodes are recommended for production, so that replenishment of metal salts during production can be avoided. In the very few cases where the use of silver anode is not desired or is not possible, platinized titanium insoluble anodes, preferably shielded insoluble anodes SIA1, can be used. In this case, a silver replenishment concentrate is required to maintain the metal concentration in the electrolyte. In this case, the consumption of the bath additives is expected to be higher due to the oxidation reactions that occur on insoluble anodes. Characteristics of the SILVERON™ GT-101 Silver DepositAppearance – The silver deposit obtained from the GT-101 silver electrolyte is white and semi-bright to mirror bright, depending on the initial surface condition of the substrate. The appearance is very similar to that of deposits from conventional cyanide silver baths. Figure 1 shows the appearance of the GT-101 silver deposits applied to connector pins with a semi-bright nickel undercoat.
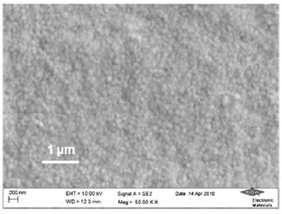
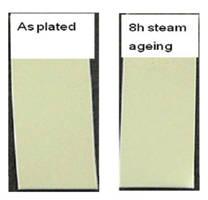
For decorative purposes, where a mirror bright deposit is required, a smooth substrate surface is very beneficial. Figure 2 shows the appearance of GT-101 silver deposits on decorative parts from a production trial. The mirror-bright, white-silver deposit can be seen from the inserted close-up view.Grain Structure – The grain structure of the GT-101 bright silver was examined using a high-resolution Field-Emission SEM. The details of the grain structure can be only observed at higher magnifications, due to the extremely fine deposit structure. An SEM image of the silver surface is presented in Figure. 3. The average grain size estimated from the SEM analysis of the surface is approximately 50 nm. Ductility – Ductility, defined as the capability of a material to deform before fracturing (cracking), is a highly desirable property for coatings in a number of applications, especially for connector application. Ductility of the silver-plated connector parts was evaluated by two connector manufacturers using common industrial test methods, including bending or crimping followed by inspection under an optical microscope. The tests demonstrate that the bright silver deposit is able to meet the industrial requests with regarding to ductility. Adhesion – In most applications, silver is either electroplated over copper and copper alloys, or over nickel undercoats. Excellent adhesion of deposits from GT-101 over copper and alloys can be achieved without using a strike layer. To ensure a good adhesion of silver over nickel undercoats, a thin silver strike layer was found to be necessary prior to silver plating For plating over nickel substrates, a novel cyanide-free silver strike solution, SILVERONTM GT-101 Silver Strike was therefore developed.Purity – Silver deposit purity is of interest for many end users. Unlike most cyanide-based silver electrolytes, the GT-101 silver electrolyte does not contain any metallic brightening agents, thus the deposit impurities are mainly associated with co-deposited carbon originating from the organic additives used in the electrolyte. Combustion IR analysis of the carbon content in the GT-101 bright silver deposit shows the high purity of the deposit, as shown in Table 2. Corrosion and Tarnish Resistance – Owing to its high reduction potential, silver possesses a naturally high resistance to oxidation, therefore under typical application conditions, silver does not form an oxide film. Even under steam ageing conditions, no visible discoloration could be observed. Figure 4 shows copper strips coated with deposits from a GT-101 silver bath before and after 8 hours steam ageing test.
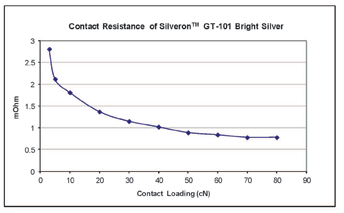
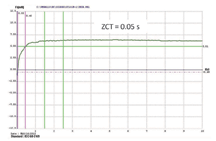
However, silver deposits are considered to be a non-noble metal finish due to their reactivity with sulphur and chlorine. It is known that when sulfide is present in the atmosphere silver coatings are prone to tarnishing, forming a Ag2S tarnish film which varies in colour between yellow, brown, blue to black, depending on the tarnish film thickness. The tarnish behaviour of the new cyanide-free silver deposit was examined under a variety of conditions. When the deposit was stored at ambient conditions (typical office environment), no visible colour change was observed over many months. An accelerated tarnish test (deposit immersed in 2% potassium polysulfide solution for 10 minutes) shows that the tarnish tendency of the silver from the new cyanide-free electrolyte is comparable to that of deposits from a conventional cyanide-based electrolyte. Several commercially available Cr6+-free, organic and inorganic anti-tarnish agents were tested and found to be effective in preventing tarnishing of the GT-101 silver deposit even under the severe test condition. Contact Resistance – Silver has the best electrical conductivity of all the metals. Electroplated silver is an excellent contact material and has been widely used for electrical/electronic connector applications, where a low contact resistance of the silver deposit is essential. The contact resistance of the new silver deposit was determined over a range of contact loadings. It can be seen from Figure 5 that a very low contact resistance (below 1 mOhm) can be achieved when the contact loading is above 40 cN. This result is very similar to that obtained on the silver deposits from conventional cyanide silver electrolytes. Solderability – Solder wetting performance of the new silver deposit was evaluated using the wetting balance technique. 2-3 microns of GT-101 bright silver was deposited on 10 x 30 x 0.2 mm copper coupons. An example of a typical wetting curve obtained for the as-plated deposit, with a Sn/Ag/Cu lead-free solder (255°C) and a rosin based RMA type flux is shown in Figure 6. Solderability of the silver plated samples was also tested after dry heat aging at 155°C for 16 hours, and also after steam ageing for 8 hours. The test results show that neither of the ageing conditions have any significant impact on the solderability of the silver. The zero crossing time (ZCT) observed at all testing conditions is well below one second, indicating that the new silver deposit has excellent solderability. Wire Bonding Capability – In wire bonding applications, several different types of silver finishes provide protection of copper substrates and allow formation of a strong bond between gold wire and silver plated substrates2. Gold wire bondability analysis of the GT-101 silver finish was carried out using an ESEC WB3088 iP wire bonding machine. Wire bonding samples were prepared by plating GT-101 silver directly over a copper substrate. Two different silver thicknesses were tested, e.g. 0.3 µm and 1.0 µm. Gold wire bonding and pull tests were performed under three different conditions:
- As plated
- Ageing at 150°C for 2 hours
- Ageing at 150°C for 2 hours, followed by plasma cleaning for 10 minutes
The wire bonding test results are given in Table 3.
Average pull force of 11.0 cN and 13.3 cN was observed on two silver coated copper samples under as-plated condition, showing that the new cyanide-free silver is capable of providing a good wire bondable surface. It is not surprising that the pull force was significantly reduced after the accelerated ageing test at 150°C for 2 hours, due to either the copper diffusion from the substrate or surface contamination. The bondability of the thicker silver coating (1.0 µm) after accelerated ageing could be fully re-established by plasma cleaning for 10 minutes, while only 50% bondability of the thinner silver coating could be recovered with the same method. When applying a thin silver layer (< 1 µm) for wire bonding application and the time to bond is relatively long, a nickel undercoat would be beneficial to prevent copper diffusion from the substrate.3
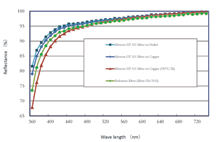
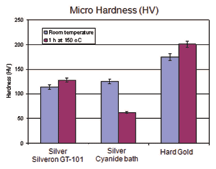
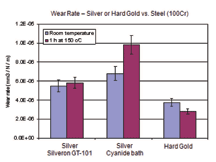
Aluminium wire bonding tests were also conducted on the same samples and extraordinarily high pull forces (> 20 cN) were showed by difference samples under all conditions. The heat treatment (150°/2h) does not have any negative influence on the Al-wire bondability of the GT-101 silver.Reflectance – Silver is one of very few metals that have high reflectivity at the wavelengths of interest for LED applications. Silver finishes from previously available cyanide-free electrolytes have, so far, not gained wide acceptance for these applications, mainly due to their limited reflectance, especially after ageing at elevated temperatures. Reflectance of GT-101 bright silver deposits was evaluated at wavelengths between 360nm and 740 nm, and compared to the reflectance of a silver deposit from a cyanide-based electrolyte which is currently used in the manufacture of LED packages. The test samples were prepared by plating 2-3 microns silver on copper and nickel substrates. The reflectance was determined both as-plated and after heat treatment at 150°C for 2 hours. In the most important wavelength range for LED (400nm–450 nm), slightly better reflectance was observed for the new silver deposit than the reference silver (from a cyanide electrolyte), as shown in Figure 7. After heat treatment of the new silver, a minor reduction in reflectance at lower wavelengths was observed, however, the value still met the requirements for the majority of LED applications. This encouraging result has initiated further evaluations of the new silver deposit for LED applications. Microhardness – Silver is a relatively soft metal. As plated, silver deposits from most cyanide-based electrolytes have a microhardness of ca. 100 HV, which is not too far below that of hard gold deposits which typically have a micro hardness of ca. 150 HV. However, electroplated silver will become progressively softer with time, even at room temperature. This softening effect is more pronounced when the deposit is exposed to elevated temperatures. The authors observed that after heating at 150°C for 1 hour, the microhardness of most silver deposits from cyanide-based electrolytes dropped up to 50% from their original values. It is believed that this characteristic was one of the main barriers to the use of silver finish in many applications. The new silver deposit does not show this softening effect and has been found to be significantly harder than deposits from conventional cyanide silver bath, especially after heat treatment, as shown in Figure 8. Friction and Wear – Generally, electrodeposited silver deposits have high coefficients of friction (COF) and poor wear resistance. Improved wear resistance or reduced COF are also desirable characteristic for electroplated silver finishes in a number of applications. In the case of connectors, COF and wear resistance of a contact finish are directly related to the connector insertion force and the maximum insertion cycles, namely durability. It is widely believed that hardness has a direct influence on the wear of a material, with wear resistance being expected to increase with increased hardness. However, this relationship is not always true. Intensive investigations of the wear behaviours of silver finishes were carried out during the development of the new silver plating process, in order to understand the failure mechanisms behind silver wear and to seek pathways to improve the wear resistance of silver finish. All wear tests were conducted without lubrication. No significant change in COF was observed for the new silver deposit when compared to a conventional cyanide silver deposit. COFs determined on both silver finishes were found to be around 1.5, which is relatively high. In comparative ball-on-plate wear tests, using silver or hard gold plated on nickel coated substrates against a stainless steel ball (100Cr) with a hardness of between 600-800 HV, improved wear resistance (reduced wear rate) was observed for the new silver deposit compared to a conventional cyanide silver deposit, probably due to the slightly increased hardness (Figure 9).
To simulate the behaviour of separable connectors, silver against silver wear tests were conducted using silver deposited on copper substrates pre-plated with nickel against the same deposit on steel balls pre-plated with nickel. A similar level of adhesive wear, so-called cold welding, was observed on both the new silver and the conventional silver, indicating that no improvement in wear resistance could be achieved by increasing deposit hardness. In this case, cold welding refers to formation of a bond between the contact interfaces that led to the removal of a large piece of silver from the deposit. Cold welding is believed to be the dominant failure mechanism in silver against silver wear. The investigations conducted so far imply that cold welding is inevitable in a wear pair of pure silver, if no lubrication is used. The prevention of cold welding in silver/silver wear is a key objective of further development. SUMMARYTo eliminate the use of cyanide in silver electroplating solutions, an improved cyanide-free silver electroplating process, SILVERON GT-101 Bright Silver, has been introduced to the market, after a number of successful industrial scale evaluation tests. The major characteristics of the electrolyte and the resulting silver deposits are sum below:
- Cyanide-free electrolyte
- Suitable for barrel, rack and reel-to-reel plating applications; with a maximum applicable current density slightly lower than that of cyanide electrolytes
- Highly stable electrolyte and consistent plating performance under production conditions
- Fine grained, bright, white silver deposit suitable for a variety of applications
- Good silver adhesion onto copper or nickel based substrates
- Low contact resistance, excellent good solderability and ductility
- Suitable for Al- and Au-wire bonding applications
- Good reflectance at wave lengths between 400-450 nm; with evaluations for LED applications in progress
- Increased deposit hardness, but comparable wear resistance to that of silver deposits from cyanide electrolytes
| Parameters | For Current Densities of 0.5 – 2 A/dm2 | For Current Densities of 2 – 15 A/dm2 |
| Silver (as Ag Metal) | 20 g/L | 40 g/L |
| Free complexing agent | 75 g/L | 75 g/L |
| SILVERON GT-101 Brightener | 10 mL/L | --- |
| SILVERON GT-101 HS Brightener | --- | 20 mL/L |
| SILVERON GT-101 HS Additive | --- | 40 mL/L |
| pH | 9.5 | 9.5 |
| Temperature | 50 °C | 50 °C |
| Anode | Pure silver anode recommended | Pure silver anode recommended |
| Cathode Efficiency | ca. 100% | ca. 100% |
| Plating Rate | 0.6 µm in 1 minute at 1 A/dm2 | 5 µm in 1 minute at 8 A/dm29 µm in 1 minute at 15 A/dm2 (>10A/dm2: jet or wire plating) |
| Silver Plating Process | Carbon Content (% w/w) | Other Co-deposited Metals | Purity |
| SILVERONTM GT-101 Bright Silver | 0.013 | No | > 99.9% Silve |
| SILVER GLOTM 3K Bright Silver(Cyanide-based reference deposit) | 0.005 | Yes | --- |
MachineGold WireBond force modeUltrasonic powerDistance of 1st (ball) and 2nd bond (wedge)Pull test | ESEC WB3088 iP25 µm Controlled force1st bond: 32%; 2nd bond: 25%1.0mm18 tests for each sample | ||
| Sample | As plated | 150°C/ 2h | 150°C/2h + pl.cl. 10' |
| CuAgAg: 0.3µm | PF [cN] | PF [cN] | PF [cN] |
| Average | 11.02 | 2.21 | 5.18 |
| Stdev | 0.97 | 0.59 | 0.65 |
| Min | 9.2 | 1.2 | 4.0 |
| Max | 13.3 | 3.8 | 6.8 |
| Range | 4.1 | 2.6 | 2.8 |
| CuAg Ag: 1.0µm | |||
| PF [cN] | PF [cN] | PF [cN] | |
| Average | 13.35 | 2.86 | 10.14 |
| Stdev | 0.61 | 0.67 | 0.66 |
| Min | 11.8 | 1.4 | 8.4 |
| Max | 14.2 | 4.4 | 11.0 |
| Range | 2.3 | 3.0 | 2.5 |
ACKNOWLEDGMENTSThe authors would like to thank Dr. Nico Onda at Altatec Microtechnologies AG, and Rikiya Shimizu at Dow Electronic Materials in Japan for their assistance in the wire bondability analysis and the reflectance measurement. REFERENCES
- S. Menard , Metal Finishing, 104 (6), pp28, 2006.
- F.S. Wu , Y. X. Hu, Y. P. Wu, B. An and J. S. Zhang, IEEE. 2005 6th International Conference on Electronic Packaging Technology, 2005
- T. Y. Lin, Michael G. Pecht, Diganta Das, Jisheng Pan, and Zhu Wenhui, IEEE TRANSACTIONS ON COMPONENTS AND PACKAGING TECHNOLOGIES , 28(2), pp.337, 2005.
ABOUT THE AUTHORSMargit Clauss has been with Dow Electronic Materials, and the former Rohm and Haas Company since 1993 and is currently working as a R&D Technologist for the Interconnection Technologies Division, Lucerne, Switzerland. She has been involved in the research and development of a variety of plating processes including copper, nickel, palladium, gold, tin, silver and alloys for electronics and automotive industries. Adolphe Foyet received the Ph.D. degree in Applied Electrochemistry from the Martin-Luther-Universität Halle-Wittenberg, Germany, in 2007. He has been postdoctoral fellow at the Eindhoven University of Technology for three years. He joined Dow Electronic Materials in 2010 and is currently working as R&D Chemist for the Interconnection Technologies Division, Lucerne, Switzerland. His research and development over the past years have been focused on corrosion protection of metals, electroplating techniques of silver, silver alloy and silver composite for connector applications. Wan Zhang received the Ph.D. degree in Physical Chemistry from Queen’s University, Ontario, Canada, in 1995. She has been with Dow Electronic Materials, and the former Rohm and Haas Company since 1996 and is presently the R&D Manager for the Interconnection Technologies division, Lucerne, Switzerland. Her research and development efforts over the last 15 years have been focused on the plating techniques of tin, palladium, nickel, silver and alloys for connector, semiconductor and circuit board applications.


