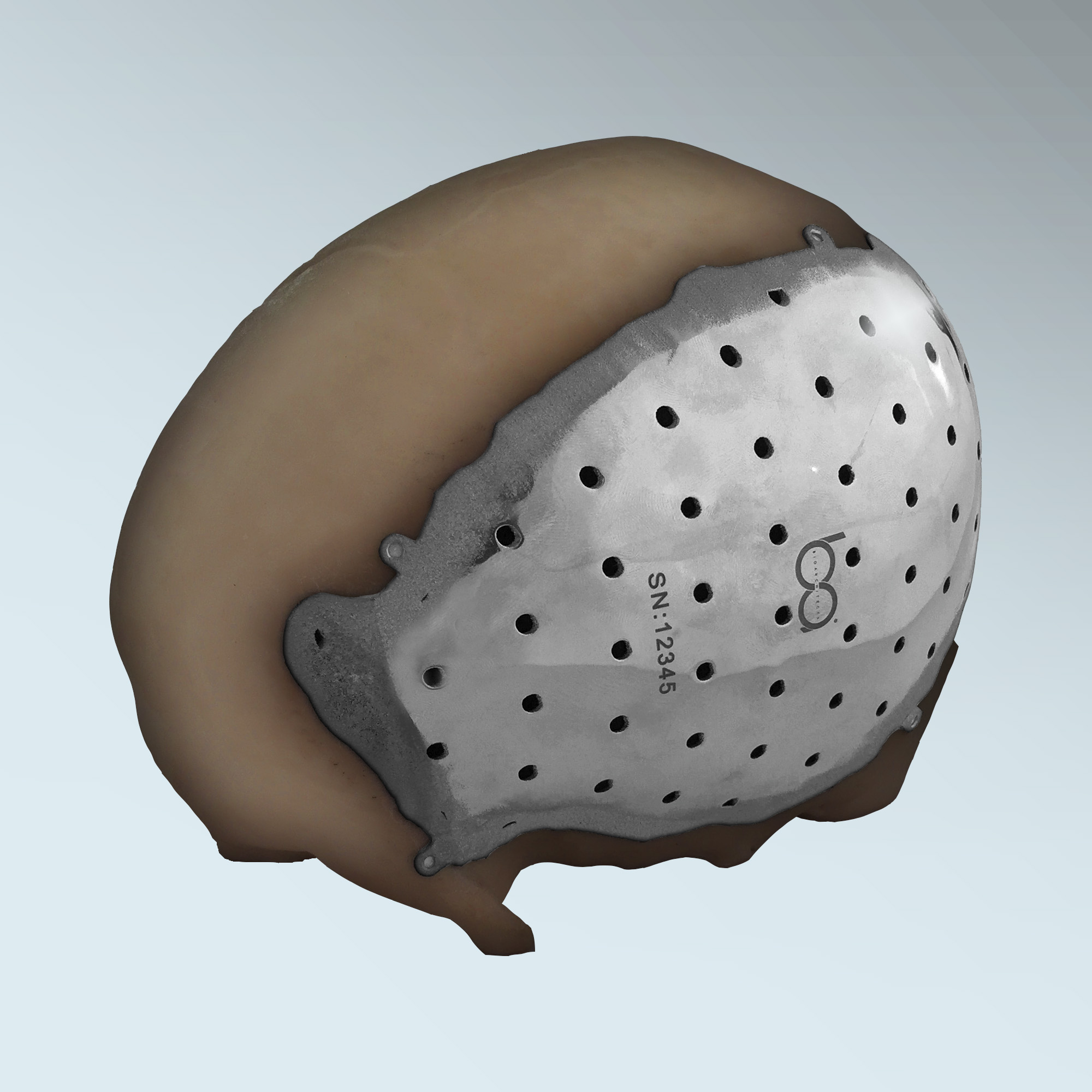
Healthcare 3D printing specialists BioArchitects has received 510(k) clearance by the U.S. Food and Drug Administration (FDA), for the company’s 3D printed bespoke titanium cranial/craniofacial plate implant.
Designed for the repair of defects in the non-loadbearing bones of the head and face, each custom designed plate is permanently attached to the skull and/or face with self-tapping titanium screws. Devices of this kind are typically used in the repair of bony defects resulting from trauma, disease, or congenital abnormalities.The plates are made using 3D printing technology from Arcam AB and a biocompatible titanium alloy.
‘We are extremely proud to contribute to what we consider another major advance in the trend toward personalized medicine,’ said Mark Ulrich, CEO of BioArchitects USA. ‘We believe that this is yet another step toward what will ultimately become the new standard of care.’
‘Arcam has been a strategic supplier to the orthopedic market for over a decade and tens of thousands of implants are made yearly from our EBM systems,’ said ’Magnus Rene, CEO, Arcam.’ It is clear that both BioArchitects and Arcam are advancing patient care with new technologies that will make a significant difference in the world of medicine for years to come.’
This story uses material from BioArchitects, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier.





