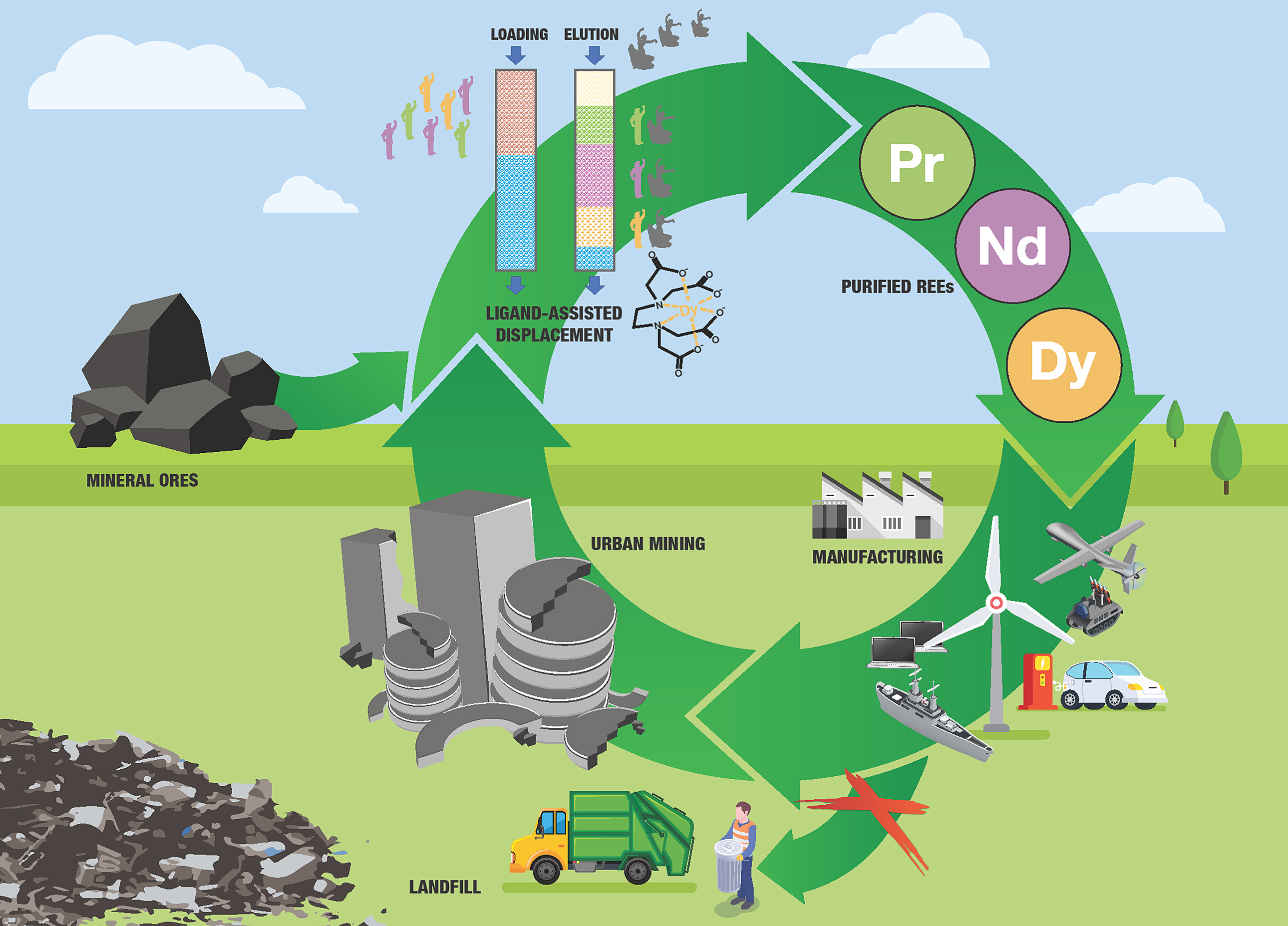
Purdue University in the US says that its extraction and purifying processes using ligand-assisted chromatography can remove and purify rare earth metals from coal ash, recycled magnets and raw ore safely, with virtually no detrimental environmental impact.
While the country is rich in rare earth metals, the current way to purify them using acid-based separation can have a detrimental environmental impact, the university said.
‘It’s a supply chain challenge with wide implications on the US economy and national security,’ said Dan Hasler, founder of Hasler Ventures, which partnered with Purdue to develop the technology. ‘We have a critically needed product and one dominant source for this product. This new patented process promises to enable US independence from the China near monopoly.’
‘Conventional methods for producing high-purity rare earth elements employ two-phase liquid–liquid extraction methods, which require thousands of mixer-settler units in series or in parallel and generate large amounts of toxic waste,’ said Nien-Hwa Linda Wang, Professor of Chemical Engineering at Purdue. ‘We use a two-zone ligand-assisted displacement chromatography system with a new zone-splitting method that is producing high-purity (>99%) metals with high yields (>99%).’
‘We continue to work diligently in the lab to learn how to adapt the ligand-assisted system to many variations we see in source material and are excited to collaborate with and assess the suitability of potential partners source material be it recycled magnets and batteries, coal ash or domestically mined ore.’
This story uses material from Purdue, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier.


