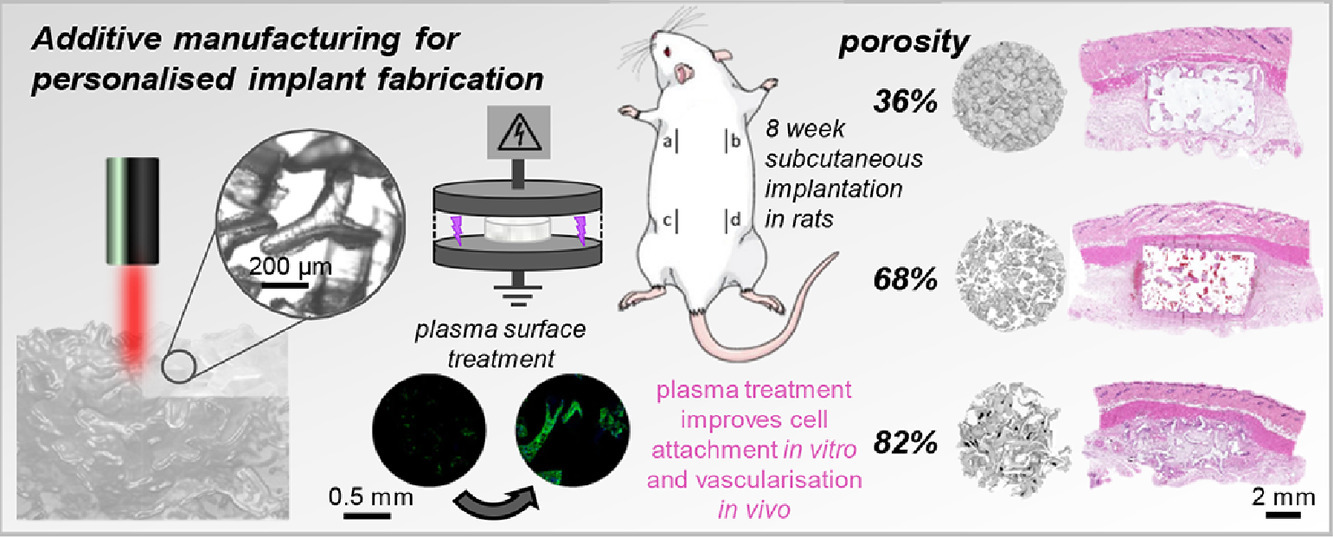
Porous high-density polyethylene (pHDPE) has been the gold standard in surgical implants for over 30 years, used in over 400,000 procedures treating bone damage or defects. Although available in a range of anatomical shapes and sizes, surgeons regularly have to trim and mold implants by hand to fit the patients’ needs. Despite the success of pHDPE implants, there is plenty of room for improving the material’s interactions with cells.
“These implants are highly rigid, which is desirable for bone reinforcement but can be problematic for soft tissue applications, and have a high risk of infection,” points out Naomi C. Paxton of Queensland University of Technology.
New techniques such as additive manufacturing offer the possibility of tailoring implants to individual patients, as well as improving other properties like porosity and surface chemistry crucial to tissue regrowth. Now Paxton and colleagues at the University of Wollongong and medical device company Anatomics have revealed how additive manufacturing and surface plasma treatment can boost the performance of pHDPE implants in supporting tissue and vascular growth [Paxton et al., Applied Materials Today 22 (2021) 100965, https://doi.org/10.1016/j.apmt.2021.100965].
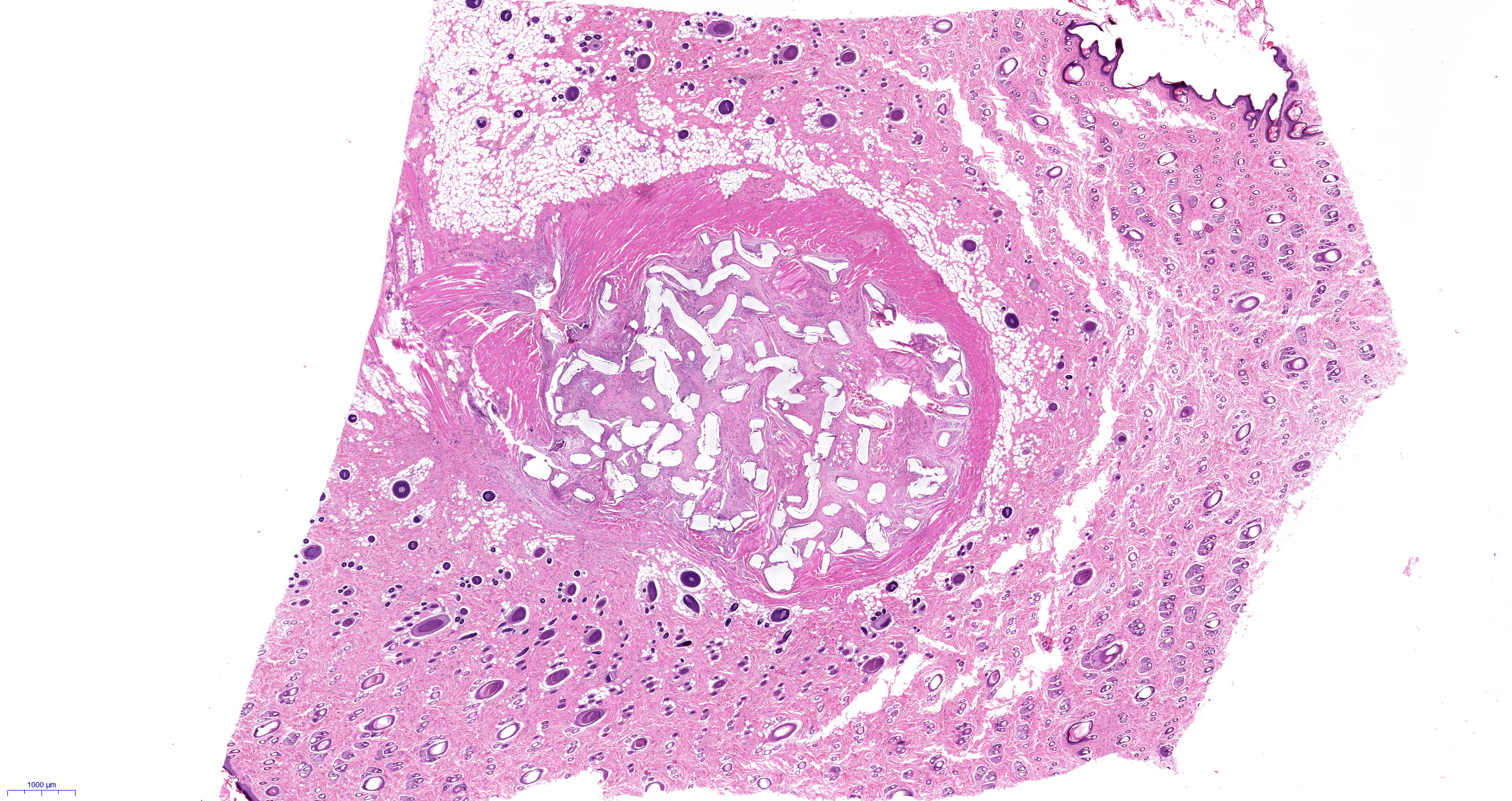
The team used laser sintering, in which a laser scans a two-dimensional (2D) pattern in a polymer powder, fusing adjacent particles together. In this way, consecutive 2D layers can be built up into complex three-dimensional (3D) structures. The researchers used proprietary star-shaped particles (StarPore®) to build highly porous scaffolds. The in vivo and in vitro performance of these novel pHDPE scaffold architectures was compared to traditional molded implants and the clinical gold-standard, MEDPOR®. Some implants were plasma treated at low temperatures in inert gases to improve hydrophilicity.
In vitro tests reveal that plasma treatment improves cell attachment 1.6-fold compared with untreated, hydrophobic pHDPE. In tests with rats, laser-sintered implants showed a marked increase (3.6-fold) in tissue ingrowth compared with traditional implants, most probably because of the higher porosity of this material. When also treated with plasma, laser-sintered scaffolds demonstrate greatly increased density of blood vessels in vivo.
“[Our] high porosity scaffolds exhibit mechanical properties more similar to native soft tissues and their high porosity [enables] rapid soft tissue and vascular ingrowth,” says Paxton.
Using additive manufacturing approaches to optimize porosity and geometry, along with surface plasma treatments, offers the promise of implants that promote rapid tissue regrowth and vascularization, improving recovery times and leading to better outcomes for patients.
“We anticipate that [our] findings will contribute to the development and implementation of 3D printing and plasma treatment in routine surgical implant manufacturing to provide customized patient-specific options for patients,” she adds. “Surgical implant manufactures [will be able] to fabricate personalized implants without the need for highly expensive one-time-use molds for patient-specific implant designs.”





