Decorative or protective paint coatings must exhibit good adhesion in order to be effective. There is no single theory that describes the property of adhesion, but there are several basic mechanisms that are known to define it. When it comes to paint systems, adhesion is provided by mainly three mechanisms: adsorption, chemical, and mechanical interlocking.
This article will review the fundamentals behind these mechanisms and reveal what characteristics of a paint system are required for good adhesion. In considering these characteristics, the paint system is viewed as a simple three-part system consisting of the paint film, the interface between the film and the substrate, and the substrate itself. Importance of Adhesion The durability and performance of paint coatings depend on two basic properties: cohesion and adhesion. Cohesion is the inner strength of a material, and it is determined by the strength of molecular forces in the bulk. It is often measured by conventional tensile and elongation tests (ASTM D638).
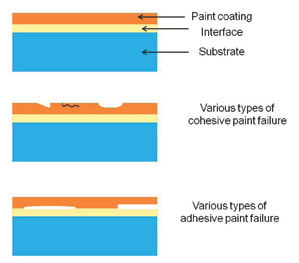
Adhesion is the strength of the bonds forming between one material and another. Illustrations of some common types of cohesive and adhesive paint failure are given in Figure 1. Cohesive failure is usually in the paint film itself (abrasion, cracking due to aging, dissolving in solvent, etc.) although it also could be within the substrate (failure of fibers in wood for example). Adhesive failure can be blister forming at the interface, lifting of the paint film, or any other situation that results from low adhesion at the interface. Adhesion test methods vary depending on the degree of information required (research, field inspection, quality control), the type of paint system and the type of substrate (rigid, flexible, weak, strong).
Both cohesion and adhesion is required for a long-lasting, protective coating. Failures related to adhesion will determine the life of the paint system. Good adhesion results when the following occurs:
- The molecules in the paint film wet or flow freely over the substrate and make intimate contact with the substrate, forming interfacial bonds (a process called adsorption)
- Chemical bonds are formed at the interface between the paint and the substrate
- The paint film penetrates the roughness on the substrate surface, resulting in mechanical interlocking once the paint dries
All three of these mechanisms do not have to be present to form good adhesion. Depending on the specific paint system, substrate, and application method, different mechanisms may be at work. However, good wetting or adsorption is universally required. Adsorption The adsorption theory states that adhesion results from molecular contact between two materials and the surface forces that develop. A bond develops from the adsorption of paint molecules on the substrate and the resulting attractive forces, usually designated as secondary or van der Waals forces. For these forces to develop, the respective surfaces must not be separated more than five angstroms in distance. Therefore, the paint film must make intimate, molecular contact with the substrate surface.
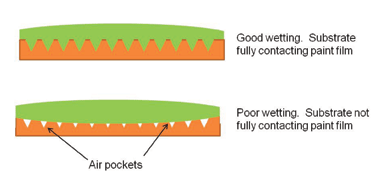
The process of establishing continuous contact between a liquid paint film and the substrate surface is known as “wetting.” Figure 2 illustrates good and poor wetting of a paint film spreading over a surface. Good wetting results when the adhesive flows into the valleys and crevices on the substrate surface; poor wetting results when the adhesive bridges over the surface irregularities. Obtaining intimate contact of the adhesive with the surface essentially ensures that interfacial flaws are minimized or eliminated. At a minimum, poor wetting causes: (1) less actual area of contact between the paint and adherend and (2) stress risers at the blisters or air pockets along the interface.
In order for good wetting to occur, the substrate must have a higher surface energy than the liquid paint film. Metals, glass, and certain polymers have a higher surface energy than most paint binders and, therefore, wetting is not a problem. However, if the substrate is contaminated with a lower surface energy material, such as shop oils, then adequate wetting will be prevented. Also, if the substrate is contaminated with loose particulate matter, the contaminant becomes a weak boundary layer which can easily fail cohesively and thereby seriously weaken the entire paint system. Certain plastic substrates, such as polypropylene, fluoroplastics, and silicone rubber, have a very low surface energy, and these substrates require some sort of surface preparation process to increase the surface energy. Common surface preparation processes are chemical, flame, and corona treatment. Chemical Bonding Certain paint systems are formulated with binders that have a functional group that can chemically bond to a compatible substrate. In these applications the formation of covalent chemical bonds occurs across the interface. These strong and durable bonds are generally the result of close contact or adsorption of the adhesive on the surface followed by a chemical reaction.
Paint systems containing reactive functional groups, such as hydroxyl or carbonyl, tend to adhere more tenaciously to substrates containing similar groups. Hydroxyl bonding is one of the reasons why epoxy and polyurethane base polymers are often used in structural paint formulations.
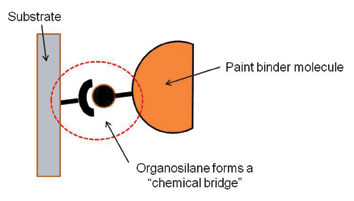
Perhaps the most widely employed example of chemical bonding in the paint industry is with adhesion promoters or coupling agents. These multifunctional chemicals provide a “molecular bridge” between the substrate and the molecules in the paint film, as illustrated in Figure 3. One end of the adhesion promoter molecule has functionality that will react with the paint, and the other end has a functionality that will react with the substrate. A strong and durable bond forms as the adhesive cures.
Organosilane is an example of a widely used adhesion promoter. They are employed as additives in paint formulations and as primers on glass and metal substrates to promote adhesion, improve moisture resistance, and reduce the potential of corrosion at the interface. Mechanical Interlocking At one time, adhesion was thought to occur only by the paint film flowing and filling pores, holes, crevices and micro-voids on the substrate. When the paint film then hardens, the paint film is held on mechanically. This theory of adhesion still predominates, especially on surfaces such as wood, concrete, or even metal and plastic. It explains why one of the most common surface treatments is abrasion or mechanical roughening.
The surface of a solid material is never truly smooth; rather, it consists of a maze of peaks and valleys. According to the mechanical theory of adhesion, in order to function properly the paint film must penetrate the irregularities on the surface, displace the trapped air at the interface, and lock-on mechanically to the substrate.
One way that surface roughness aids in adhesion is by increasing the total contact area between the paint and the adherend. If interfacial or intermolecular attraction is the basis for adhesion, increasing the actual area of contact will increase the total energy of surface interaction by a proportional amount. Thus, the mechanical interfacing theory generally teaches that roughening of surfaces is beneficial because it: (1) gives “teeth” to the substrate and (2) increases the total effective area over which the forces of adhesion can develop.
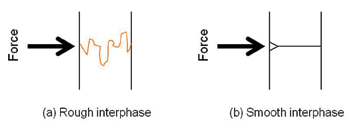
Another benefit of mechanical interlocking is that a rough surface will provide a crack propagation barrier. Notice that in Figure 4, as a wedge is driven into the edge of a smooth interface, little energy dissipation is required to separate the adherends, and a clean separation is possible. In this case, the substrates may simply “unzip”. However, if there is surface roughness, then a tortuous interface between the adhering materials will act as “path breaks” between the separating adherends. These excursions dissipate energy and increase the resulting strength of the joint.
Thus, there are many cases where the physio-chemical forces of adhesion and mechanical interlocking forces are working together in the same joint. In these cases, the practical work of adhesion is equal to the work developed by adhesion mechanisms (van der Walls forces) in addition to the work developed by mechanical mechanisms (elastic deformation). BIO Edward M. Petrie is the sole proprietor of EMP Solutions, a Cary, N.C.–based consulting firm focused on solving problems in the adhesives and sealants industry. He also works as a technical expert for SpecialChem. Visit www.specialchem4adhesives.com for more details.
