Metal processors should be familiar with some basic information about, nitric oxides, or NOx,as it will likely be regulated in their plants in the very near future, if it is not already. Fortunately, there are many well-established methods for controlling and minimizing NOx.
What is NOx?
Most of the world's nitrogen occurs naturally in the atmosphere as an inert gas contained in air, which consists of approximately 78% N2 by volume. NOx refers to oxides of nitrogen. These generally include nitrogen monoxide, also known as nitric oxide (NO), and nitrogen dioxide (NO2). They may also include nitrous oxide (N2O), also known as laughing gas, as well as other less common combinations of nitrogen and oxygen, such as nitrogen tetroxide (N2O4) and nitrogen pentoxide (N2O5).
The U.S. EPA defines nitrogen oxides as "all oxides of nitrogen except nitrous oxide."1 In most high-temperature heating applications, the majority of the NOx exiting the exhaust stack is in the form of nitric oxide (NO).
What's Wrong with NOx?
Health hazards: Nitric oxide is poisonous to humans and can cause irritation of the eyes and throat, tightness of the chest, nausea, headache, and gradual loss of strength. Prolonged exposure to nitric oxide can cause violent coughing, difficulty in breathing, and cyanosis; it could be fatal. NO2 is a reddish-brown, highly reactive gas that has a suffocating odor and is a strong oxidizing agent. It is highly toxic and hazardous because of its ability to cause delayed chemical pneumonitis and pulmonary edema. NO2 vapors are a strong irritant to the pulmonary tract.
Ground level ozone: Ozone (O3) is present in the upper atmosphere and is desirable as it shields the earth against high-intensity ultraviolet rays from the sun. However, ozone in the lower atmosphere is undesirable. There, NO reacts with oxygen to form ozone, in addition to NO2:
NO + HC + O2 + sunlight → NO2 + O3
Ozone is also a health hazard that can cause respiratory problems in humans. It is an irritant to the eye, nose, and throat. Ozone can cause damage to plants including crops, and deterioration of textiles and other materials.
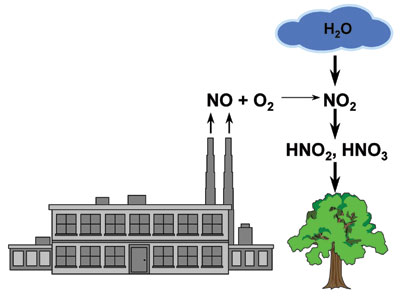
Acid rain: Rain is effective at removing NO2 from the atmosphere. However, NO2 decomposes on contact with water to produce nitrous acid (HNO2) and nitric acid (HNO3), which are highly corrosive (see Figure 1). NO is the predominant form of NOx produced in industrial combustion processes.
It reacts with O2 in the atmosphere to form NO2. When NO2 forms in the atmosphere and comes into contact with water in the form of rain, nitric acid is formed. Acid rain is destructive to anything it contacts, including plants, trees, and man-made structures like buildings, bridges, and the like.
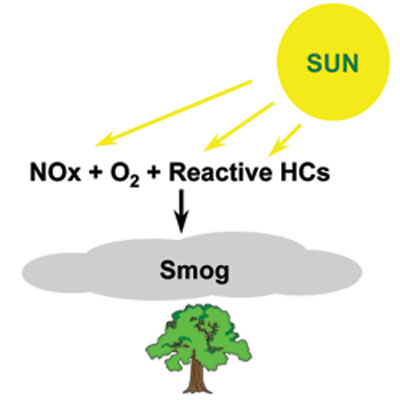
Smog: Another problem with NO2 is its contribution to smog. Smog is the combination of smoke and fog and occurs where there are high concentrations of pollutants combined with fog. Smog impairs visibility through the atmosphere.
When sunlight contacts a mixture of NO2 and unburned hydrocarbons in the atmosphere, photochemical smog is produced (see Figure 2).
How Does NOx Form?
There are three generally accepted mechanisms for NOx formation: thermal NOx, prompt NOx, and fuel NOx. Thermal NOx is formed by the high-temperature reaction (hence, the name thermal NOx) of nitrogen with oxygen, by the well-known Zeldovich mechanism:
N2 + O2 → NO, NO2
Thermal NOx increases exponentially with temperature. Above about 2,000°F (1,100°C), it is generally the predominant mechanism in combustion processes, making it important in most high-temperature heating applications. It means this mechanism becomes more important when air preheating of the combustion air is used, which normally increases the flame temperature that leads to increased NOx.
Prompt NOx is formed by the relatively fast reaction (hence, the name prompt NOx) between nitrogen, oxygen, and hydrocarbon radicals:
CH4 + O2 + N2 → NO, NO2, CO2, H2O, trace species
In reality, this very complicated process consists of hundreds of reactions and dozens of species. Prompt NOx is generally an important mechanism in lower- temperature combustion processes, but is generally much less important compared to thermal NOx formation at the higher temperatures found in many industrial combustion processes.
Prompt NOx becomes more important under fuel rich conditions.
Fuel NOx is formed by the direct oxidation of organo-nitrogen compounds contained in the fuel (hence, the name fuel NOx) and is given by the overall reaction:
RxN + O2 → NO, NO2, CO2, H2O, trace species
Fuel NOx is not a concern for high-quality gaseous fuels like natural gas or propane, which normally have no organically-bound nitrogen. However, fuel NOx may be important when oil (e.g., residual fuel oil), coal, or waste fuels are used, which may contain significant amounts of organically bound nitrogen.
How is NOx Controlled?
Before air quality regulations, the flue gases from combustion processes were vented directly into the atmosphere. As air quality laws tightened and public awareness increased, the industry began looking for new strategies to curb NOx emissions. The general strategies for reducing NOx are discussed next.2
A summary of NOx control techniques developed for the U.S. EPA can be found in EPA Report 450/1-78-001.3 Table I shows a summary of common NOx control technologies, including combustion modifications, process modifications, and post-treatment.4 The four basic control strategies are:
Pretreatment: The first NOx reduction strategy is referred to as pretreatment, which is a preventative technique to minimize NOx generation. In pretreatment, the incoming feed materials (fuel, oxidizer, and/or material being heated) are treated in such a way as to reduce NOx.
Some examples include fuel switching or treatment, using additives, oxidizer switching, and product switching or treatment.
For example, partially or completely substituting natural gas for fuel oil can often significantly reduce NOx emissions by reducing the amount of nitrogen in the fuel. Switching from air to pure oxygen for combustion eliminates most, if not all, of the nitrogen from the process so NOx is minimized or eliminated. Removing nitrogen that may be present in the feed materials may also reduce NOx formation.
Process modification: There are a number of techniques that may be employed to change the existing process in such a way as to reduce NOx emissions. For example, if the mass of NOx emitted from a plant is too high, one alternative is to reduce the firing rate. The reduction in NOx is proportional to the reduction in firing rate as less fuel is burned and, therefore, less NOx is formed. However, production is also reduced as well.
Another example is to replace some or all of the gas-fired equipment with electrically heated units that do not produce any NOx emissions at the point of use. However, the operating costs often increase as electricity is usually more expensive than fossil fuels in heating applications.
Another method is to improve the thermal efficiency of the process so less fuel is consumed per unit of production. This not only reduces pollution emissions, but operating costs as well. In some special cases, it may be possible to switch the material being heated to one that requires less energy to process. Some of these process modifications are radical and expensive and not often used except under certain circumstances.
Combustion modification: Another strategy for reducing NOx is known as combustion modification, where NOx formation is minimized by changing the combustion process. There are numerous methods that have been used to accomplish this.
Reducing the level of air preheat, if present, can significantly reduce NOx. However, this also reduces thermal efficiency and productivity. Reducing the amount of excess air is a good way to reduce NOx and increase thermal efficiency. This includes reducing air infiltration into the combustion process.
However, reducing the excess air level too much can increase carbon monoxide, which is another regulated pollutant.
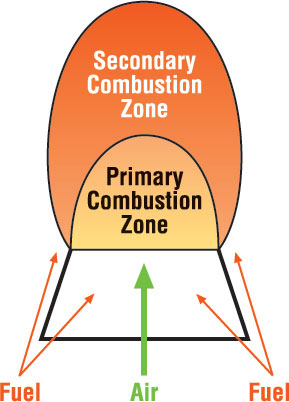
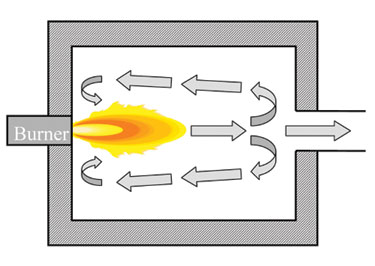
A popular method is to replace existing burners with low NOx designs.5 These incorporate a variety of techniques for reducing NOx such as air and fuel staging (see Figure 3), internal furnace gas recirculation (see Figure 4), water or steam injection, and ultra-lean premixing.
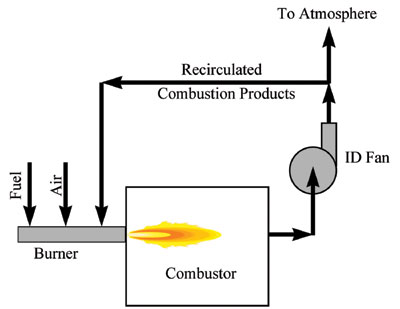
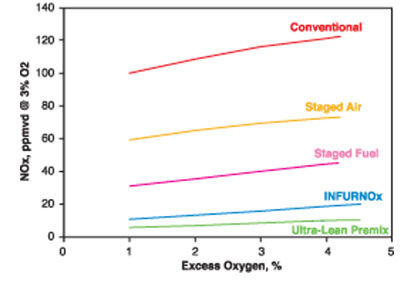
External flue gas recirculation is another common technique for reducing NOx (see Figure 5). Most of these techniques involve reducing the peak flame temperatures that produce high levels of NOx. Figure 6 shows an example of how new generations of burner designs continue to reduce NOx emissions.
Post-treatment: Another strategy for minimizing NOx is known as post-treatment where NOx is removed from the exhaust gases after it has already been formed in the combustion chamber.
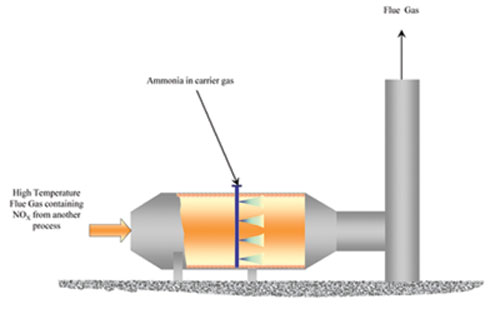
The general strategy is to use a reducing agent, such as CO, CH4, other hydrocarbons, or ammonia, to remove the oxygen from the NO and convert it into N2 and O2. Often, some type of catalyst is required for the reactions.
Two of the most common methods of post-treatment are selective catalytic reduction (SCR) and selective noncatalytic reduction (SNCR). SCR is generally used instead of SNCR (see Figure 7) when higher NOx reduction is required.
A catalyst is a substance that causes or speeds up a chemical reaction without undergoing a chemical change itself. One of the advantages of post-treatment is that multiple exhaust streams can be treated simultaneously, thus achieving economies of scale.
Most of the post-treatment methods are relatively simple to retrofit to existing processes. However, most are also fairly sophisticated and are not trivial to operate and maintain in industrial environments. For example, the catalytic reduction techniques require a catalyst that may become plugged or poisoned fairly quickly by dirty flue gases. Post-treatment methods are often capital intensive, and they usually require halting production if there is a malfunction of the treatment equipment.
| Technology | Approximate Reduction | Approximate Emissions (lb/MMBTU) |
| Standard burners | Base case | 0.14 |
| Low NOx burners (LNB) | 60% | 0.06 |
| Ultra-low NOx burners (ULNB) | 80–95% | 0.007–0.03 |
| Flue gas recirculation | 55% | 0.025 |
| Selective noncatalytic reduction (SNCR) | 40% | 0.033–0.085 |
| Selective catalytic reduction (SCR) | 90–97% | 0.006–0.015 |
| Adapted from Bradford et al.4 |
Conclusions
NOx is a regulated pollutant formed in nearly all industrial combustion processes. It can generally be easily controlled using a variety of proven strategies. The most cost-effective technique tends to be combustion modification, such as using low NOx burners. In virtually all cases, proper care must be taken to carefully operate and maintain the equipment to keep it within the desired range for low emissions. Suitable instrumentation, such as gas analyzers for measuring O2 and NOx in the exhaust products, is recommended to ensure the equipment is operating according to specifications.
This will help metal processors continue to be environmentally friendly and within compliance of their air permits.
References
- Office of the Federal Register, 60.2 Definitions, U.S. Code of Federal Regulations Title 40 Part 60, Washington, D.C.: U.S. Government Printing Office: 2001.
- Baukal, C.E., Industrial Combustion Pollution and Control, Marcel Dekker, New York: 2004.
- U.S. EPA, Control Techniques for Nitrogen Oxides Emissions from Stationary Sources, EPA Report 450/1-78-001, Washington, D.C., U.S. Environmental Protection Agency: 1978.
- Bradford, M., Grover, R., and Paul, P., "Controlling NOx Emissions– Part 1," CEP Magazine, 98(3):42-46: 2002.
- Baukal, C.E. (ed.), Handbook of Industrial Burners, CRC Press, Boca Raton, FL: 2004.
Bio
Charles Baukal is the director of R&D for John Zinc Co. in Tulsa Okla.






